the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
A simple, versatile approach for coupling a liquid chromatograph and chemical ionization mass spectrometer for offline analysis of organic aerosol
Andre F. Schaum
Kelvin H. Bates
Kyung-Eun Min
Faith Myers
Emmaline R. Longnecker
Manjula R. Canagaratna
Mitchell W. Alton
A method is described for coupling a high-performance liquid chromatograph (HPLC) and chemical ionization mass spectrometer (CIMS) for the offline analysis of organic aerosol. It employs a nebulizer interface and the Vaporization Inlet for Aerosols (VIA), allowing for the transmission of analytes from the HPLC eluent into the CIMS inlet. Performance of the HPLC-VIA-CIMS system was assessed through the analysis of carboxylic acid standards, environmental chamber-generated secondary organic aerosol (SOA) formed from the ozonolysis of α-pinene, and ambient OA collected in an urban setting. Chromatographic peak shapes were retained through nebulization and evaporation, providing baseline-resolved separation of C6–C18 carboxylic acids and generating molecular-level detail that is not attainable using HPLC or CIMS alone. Instrument response was found to be linear (R2 > 0.97) over an order of magnitude (0.2–3.0 nmol or 2–30 nmol on column) for each of the 12 standards. Analysis of α-pinene ozonolysis SOA achieved isomer-resolved detection of both monomer and dimer reaction products and, through the use of a diode array detector (DAD), illustrated the preservation of chromatographic peak shape through nebulization and evaporation. The HPLC-VIA-CIMS instrument also shows potential for quantitative analysis, provided that authentic standards can be purchased or synthesized, and semi-quantitative analysis of UV-absorbing compounds such as nitrates and carboxylic acids by using a DAD. The system is compatible with small sample quantities (e.g., 30 µg of α-pinene ozonolysis SOA), allowing for detailed molecular characterization of field-collected SOA, including the identification of several monoterpene oxidation products.
- Article
(1608 KB) - Full-text XML
-
Supplement
(1250 KB) - BibTeX
- EndNote
Atmospheric aerosols, particularly those smaller than 1 µm in diameter, present direct risks to human health and can influence the global climate through their contributions to radiative forcing (Pöschl, 2005). These effects are highly dependent on the chemical and physical properties of aerosol particles (Mauderly and Chow, 2008; Shrivastava et al., 2017), highlighting the need for analytical techniques that can provide detailed information about their composition. Characterization of organic aerosol at a molecular level, however, is challenging due to the chemical complexity (> 104 known compounds) produced by the oxidation of volatile organic compounds (VOCs) to low-volatility products that form secondary organic aerosol (SOA; Goldstein and Galbally, 2007). The constituents of SOA are often multifunctional, containing combinations of nitrate, hydroxyl, carboxyl, carbonyl, peroxide, and other oxygen-containing functional groups that influence the physicochemical properties of the aerosols (Kroll and Seinfeld, 2008; Ziemann and Atkinson, 2012).
Real-time analysis of organic aerosol (OA) composition has most often been conducted using the Aerodyne aerosol mass spectrometer (AMS; Canagaratna et al., 2007), in which particles are sampled into a high-vacuum chamber and vaporized by impaction on a hot surface. The vapor is then analyzed by electron ionization mass spectrometry (EIMS). Compared to chemical ionization mass spectrometry (CIMS), the AMS has the advantage that it can be used to quantify the major OA components (e.g., oxidized, hydrocarbon-like, and biomass-burning OA) through the use of appropriate calibration and data analysis methods. Its disadvantage is that EI (combined with the 600 °C vaporization temperature) leads to extensive fragmentation that usually prevents the assignment of compound molecular formulas. The fragmentation problem can usually be overcome by the use of CIMS, with the trade-off being the loss of OA quantitation, except for single compounds when authentic molecular standards are available. In this case, particles are thermally desorbed before entering the CIMS ion–molecule reaction region, usually by passing through a heated tube at significantly lower temperatures than those used in the AMS. This approach has been employed with a large variety of CIMS reagent ions, for example, H+(H2O)n (Warscheid and Hoffmann, 2001); H+(H2O)2, H+(CH3OH)2, NO+, O, O, F−, SF (Hearn and Smith, 2004); H+(H2O)n, I−(H2O)n, CH3C(O)O− (Aljawhary et al., 2013); H3O+ (Eichler et al., 2015); and NO (Häkkinen et al., 2023). These provide a wide range of ionization specificity and sensitivity that can be an advantage or disadvantage depending on the compounds of interest. Although effective at analyzing real-time changes in aerosol composition, these approaches usually fail to differentiate between isobaric species that commonly exist in SOA. One way to address this limitation is by coupling online ion mobility spectrometry with mass spectrometry (IMS-MS; Krechmer et al., 2016), but the use of this approach for atmospheric chemistry studies is in its infancy because, until recently, technical challenges have hindered the development of a commercial instrument with a reliable gas-phase ion source. Instead, methods employed to couple separation with MS have all involved semi-online or offline analysis.
The simplest of these has employed sample preconcentration and programmed thermal desorption for semi-online analysis, as with the thermal desorption particle beam mass spectrometer (TDPBMS; Tobias and Ziemann, 1999), thermal desorption chemical ionization mass spectrometer (TDCIMS; Voisin et al., 2003), and filter inlet for gases and aerosols (FIGAERO; Cai et al., 2023). However, the resolution achieved by these methods is typically poor compared to other separation methods such as chromatography.
Gas chromatographic (GC) separation has been used in combination with CIMS instruments to resolve isomers and other closely related compounds in the atmosphere. Previous work includes the analysis of VOCs by proton-transfer-reaction mass spectrometry (PTR-MS; Warneke et al., 2003) and by I− (Robinson et al., 2024) and CF3O− (Vasquez et al., 2018) CIMS as well as aerosol by coupling a thermal desorption aerosol gas chromatograph (TAG) with I− CIMS (Bi et al., 2021a, b). A disadvantage of using GC to separate OA constituents is the low transmission of multifunctional compounds through the column due to irreversible adsorption and/or thermal decomposition. However, this can be ameliorated to some degree by the chemical derivatization of hydroxyl, carbonyl, carboxyl (Yu et al., 1998, 1999), and hydroperoxide (Docherty et al., 2004) groups to increase analyte stability and volatility and by the use of specialized GCs that utilize reduced pressure (Vasquez et al., 2018) or cryo-trapping (Robinson et al., 2024).
An alternative for compounds that are not amenable to the high temperatures used in GC analysis is high-performance liquid chromatography (HPLC), which is generally conducted at ambient temperatures and offers flexibility in the choice of mobile phase and gradient elution for optimizing separations. HPLC has previously been used for the analysis of SOA generated in environmental chambers and ambient SOA by coupling with several types of ionization and MS detection methods (Hildmann and Hoffmann, 2024). Electrospray ionization (ESI) is most commonly used (Hildmann and Hoffmann, 2024), although extractive electrospray ionization (EESI; Schueneman et al., 2024), atmospheric pressure chemical ionization (APCI; Hoffmann et al., 1998; Reinnig et al., 2008), atmospheric pressure photoionization (APPI; Ruiz-Jiménez et al., 2012), and EI (Yeh and Ziemann, 2014) have also been employed. However, while these approaches are capable of separating complex mixtures and generating high-resolution mass spectra, the devices used to nebulize, vaporize, and ionize OA components following HPLC fractionation can suffer from matrix effects in ESI and EESI, and they are generally integrated in such a way as to allow limited opportunity for independently and easily varying the ionization method. This is a considerable drawback, considering the wide variety of CIMS reagent ions now available for selectively ionizing different compound classes and achieving exceptionally high detection sensitivity, the recently developed capability for rapidly switching among multiple reagent ions (Alton et al., 2024) for more comprehensive CIMS analysis, and the advantage of being able to analyze gas- and particle-phase compounds with the same CIMS methods.
Here, we describe a simple, versatile approach for coupling an HPLC to an I− CIMS (I-CIMS) using a commercially available nebulizer and an Aerodyne Vaporization Inlet for Aerosols (VIA; Zhao et al., 2024) for the separation and analysis of SOA. The performance of the HPLC-VIA-CIMS system is first evaluated using a mixture of simple and functionalized carboxylic acid standards and then through analysis of environmental chamber-generated SOA and an aerosol sample collected from an urban environment. Lastly, further applications and possible improvements to the system are discussed.
2.1 Chemicals
The following chemicals were used throughout the experiments described below: methanol (HPLC grade, Fisher Chemical), acetonitrile (HPLC grade, Fisher Chemical), Milli-Q water (18.2 MΩ, < 5 ppb TOC), N2 (ultrahigh purity, Airgas), glacial acetic acid (ACS grade, Fisher Chemical), (1R)-(+)-α-pinene (99 %, Sigma Aldrich), cis-pinonic acid (98 %, Aldrich), and trans-norpinic acid (Sigma Aldrich). Ozone was generated using a calibrated Ozone Engineering LG-7 ozone generator and pure O2 (industrial grade, Airgas). The organic acid standards had purities of at least > 97 % and included adipic acid, suberic acid, sebacic acid, dodecanedioic acid, palmitic acid, stearic acid, and 16-hydroxypalmitic acid from TCI America; pimelic acid, azelaic acid, and 12-hydroxystearic acid from Thermo Scientific; undecanedioic acid and 2-hydroxypalmitic acid from MedChemExpress and Ambeed, Inc.
2.2 Environmental chamber experiment
The ozonolysis of α-pinene without the use of an OH scavenger was carried out in a 6.7 m3 Teflon chamber at an ambient pressure (630 Torr) and temperature (22 °C) filled with dry air (< 1 % RH, < 5 ppbv hydrocarbons) from two AADCO clean air generators. Approximately 500 ppb of ozone was added to the chamber using a calibrated glass bulb, and the concentration was verified using a 2B Technologies model 202 ozone monitor. Immediately following the ozone addition, 300 ppb of α-pinene was added using a custom-built vaporizer (Finewax et al., 2020). A Teflon-coated fan was used throughout each addition to mix the chamber air. The reaction was allowed to proceed for 40 min before sampling the SOA onto two pre-weighed Millipore Fluoropore PTFE filters (0.45 µm pore size) at a flow rate of 14.2 L min−1 each for 1 h. After sampling, the filters were weighed, extracted twice with 6 mL acetonitrile, evaporated under dry N2, and stored at −10 °C until analysis. Extraction efficiency was determined to be ∼ 98 % based on the measured filter mass difference pre- and post-extraction. Prior to analysis, the SOA was reconstituted in methanol to achieve a total mass concentration of 2 mg mL−1.
2.3 Field-collected OA
Ambient OA was collected in October 2024 at the Denver, Colorado ASCENT site located approximately 4 km from downtown Denver in a suburban setting that also contains commercial activities (La Casa; ASCENT, 2024). Air quality at this site is influenced by anthropogenic and biogenic sources, including oil and gas operations and biomass burning. The OA sample was collected using an MCV high-volume sampler (model CAV-A/mb) affixed to a PM1 sampling head operated at a flow rate of 30 m3 h−1. The sampler collected aerosol onto a single Pall Tissuequartz filter (P.N. 7204) between the hours of 04:00 and 10:00 Mountain Daylight Time over the course of 1 week for a total of 42 h. At the end of the sampling period, the filters were transported on ice and stored at −10 °C until extraction. A full description of the extraction method and additional sampling details can be found in the Supplement.
2.4 Instrumentation
2.4.1 High-performance liquid chromatography
Analyses were performed using a Shimadzu Prominence HPLC system with a Nexera X2 SPD-M20A diode array detector (DAD) and an Agilent XBD-C8 column (150 × 4.6 mm, 5 µm). The aqueous mobile phase (A) consisted of 95 % water, 5 % acetonitrile, and 0.05 % glacial acetic acid (), whereas the organic mobile phase (B) was 95 % methanol, 5 % acetonitrile, and 0.05 % glacial acetic acid (). The addition of acetonitrile to the mobile phases serves as a dopant to suppress the formation of I• (H2O)− clusters and to reduce the humidity dependence of the I-CIMS performance (Riva et al., 2024). Due to the large amount of water present in the HPLC eluent, omitting this addition results in significant depletion of the reagent ion and reduces instrument sensitivity. Although a mobile-phase concentration of 5 % was determined to be sufficient for the analyses described in this study, further optimization could be done to maximize instrument performance.
Mobile phase additives are commonly used in reverse-phase HPLC to modify eluent pH and improve peak shape and retention of ionizable analytes (e.g., carboxylic acids; Schwarzenbach, 1982). However, analyte detection issues arise with typical pH modifiers such as formic acid and trifluoroacetic acid due to the relatively high sensitivity of I-CIMS to these acids. Acetic acid has been reported to have an I-CIMS sensitivity that is 30 times lower than that of formic acid (Lee et al., 2014) and was thus chosen as a pH modifier to improve the chromatography while limiting reagent ion depletion. Although the addition of a weak acid primarily served to improve the peak shapes of carboxylic acids in this study, it has been shown that lowering mobile-phase pH can also improve the retention and peak shapes of basic analytes through ion pairing effects (Lobrutto et al., 2001). It is also worth noting that because compound evaporation and ionization are spatially separated in I-CIMS instruments (unlike in ESI), I-CIMS is not susceptible to ionization suppression caused by non-ideal electrospray conditions required to counteract the high conductivity and surface tension imparted to droplets by the organic acids (Apffel et al., 1995).
Gradient elution methods were used for analyte separation at a flow rate of 1 mL min−1. The gradients used for the carboxylic acid standards, α-pinene SOA, and field-collected OA, respectively, were as follows: 20 % B for 1.5 min, increase to 100 % B over 12.5 min and hold for 5.5 min, return to initial conditions; 10 % B for 1.5 min, increase to 80 % B over 18 min, increase to 100 % B over 0.5 min and hold for 3 min, return to initial conditions; 5 % B for 1.5 min, increase to 100 % B over 17.5 min and hold for 5 min, return to initial conditions. The HPLC-DAD chromatograms were baseline corrected using injections of solvent blanks.
2.4.2 VIA-CIMS
The I-CIMS instrument used for SOA analysis was previously described by Devault et al. (2022a). Briefly, a Tofwerk ion mobility spectrometry-time-of-flight (IMS-TOF) mass spectrometer (Krechmer et al., 2016) with the IMS cell replaced by an iodide chemical ionization source was mounted with a VIA without the honeycomb activated carbon denuder to minimize analyte loss (Zhao et al., 2024). An additional vacuum line connected to a needle valve was installed immediately downstream of the VIA to increase the flow rate through the system above the I-CIMS inlet flow of 2 L min−1. For all experiments, the VIA was operated at a temperature of 200 °C and a flow rate of 3.5 L min−1 to limit wall losses and thermal decomposition by reducing analyte residence time (Zhao et al., 2024). The I-CIMS data were processed using Tofware version 4.0.1, and chromatographic peak fitting was performed using the Multipeak Fitting package in IGOR Pro 9 (WaveMetrics).
2.4.3 Nebulizer interface
The HPLC and I-CIMS inlet were coupled using a home-built nebulizer interface consisting of an electrospray nebulizer (Agilent EN10501) that was mounted in a three-port 300 mL glass bulb with a rubber stopper (Fig. 1). This bulb volume was chosen to maximize the distance between the nebulizer tip and the bottom of the bulb in order to reduce the impaction of the nebulizer spray while not affecting peak broadening, as shown below. The nebulizer was supplied with N2 at a pressure of 25 psi, resulting in an N2 flow of 1.5 L min−1, and the HPLC eluent was introduced at a flow rate of 1 mL min−1. The total flow through the nebulizer bulb was adjusted to 3.5 L min−1 by flowing dry N2 through a port orthogonal to the nebulizer spray; the N2 flow rate was chosen to be consistent with the manufacturer's recommendations for VIA performance and was also found to optimize analyte signal in preliminary testing (Fig. S2 in the Supplement). To prevent large droplets from reaching the VIA, a trap consisting of a 30 mL gas bubbler (Chemglass, CG-1821-01) with the glass frit removed was connected to the outlet of the glass bulb using 8 cm of conductive silicone tubing (4.5 mm ID, 9 mm OD). The gas bubbler outlet was connected to the VIA with 1 m of conductive PFA (cPFA) tubing ( in. ID × in. OD, Fluorotherm F015202G).
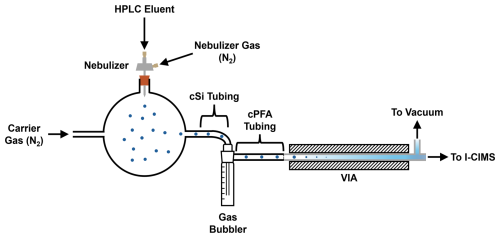
Figure 1Schematic of nebulizer interface and VIA. The HPLC eluent flowing at 1 mL min−1 is nebulized into a glass bulb, and the resulting solvent/analyte droplets are carried to the VIA by a total N2 flow of 3.5 L min−1. A gas bubbler (glass frit removed) prevents large droplets from reaching the VIA. The remaining droplets are vaporized in the VIA and proceed into the I-CIMS.
3.1 Separation and detection of carboxylic acids
Since simple and functionalized organic acids are often found in SOA and have high I-CIMS sensitivities (Lee et al., 2014), a selection of monocarboxylic acids, dicarboxylic acids, and hydroxycarboxylic acids were chosen as model analytes to evaluate the HPLC-VIA-CIMS performance. The organic acid standards (Table 1) were prepared in a solution of methanol at concentrations of 0.1 mM for the dicarboxylic acids and hydroxycarboxylic acids and 1 mM for palmitic acid and stearic acid. Figure 2 shows an HPLC-VIA-CIMS chromatogram of a 15 µL injection (1.5–15 nmol) of the standards separated over 18 min. In general, retention time is inversely correlated with analyte polarity for reverse-phase HPLC separations, meaning that more highly functionalized compounds will elute earlier than those with fewer functional groups. This is clearly observed in Fig. 2 with the dicarboxylic acids eluting first, followed by the hydroxycarboxylic acids and the monocarboxylic acids. It is important to note the baseline-resolved separation of 16- and 2-hydroxypalmitic acid, which are indistinguishable by molecular formula alone and are unlikely to be separated with similar resolution by thermal desorption methods. The increase in dicarboxylic acid signal with increasing carbon number up to sebacic acid in Fig. 2 is the opposite of the trend observed by Lee et al. (2014) for direct evaporation of succinic, glutaric, adipic, and azelaic acid into an I-CIMS. This suggests that the signals observed here may have been influenced by the HPLC and/or VIA, perhaps due to differences in the composition of solvent drops exiting the HPLC or other factors that might affect evaporation, decomposition, and wall loss in the VIA. This could be investigated in the future by, for example, using isocratic HPLC, varying the vaporizer temperature, and measuring the aerosol size distribution.
Table 1Calibration curve linearity and sensitivity for 12 carboxylic acid standards. Compounds are arranged in ascending order of retention time.
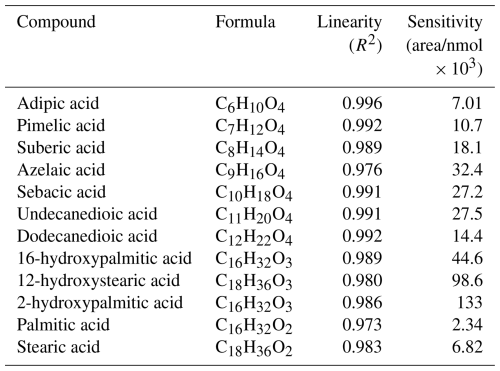
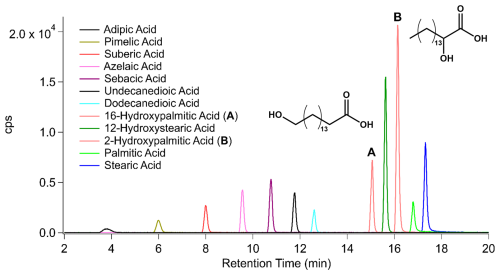
Figure 2HPLC-VIA-CIMS extracted ion chromatogram of a mixture containing 12 organic acid standards. The injection quantity was 1.5 nmol for each standard except for palmitic acid and stearic acid, which were 15 nmol each. The structures of 16-hydroxypalmitic acid (A) and 2-hydroxypalmitic acid (B) are included for clarity.
An additional advantage over separation by thermal desorption is the greater predictability of reverse-phase HPLC separation based on molecular structure. For example, when using a column that separates compounds through hydrophobic interactions (e.g., a C8 column), the amount of interaction between analytes and the stationary phase can be inferred by both the number of polar functional groups and their relative positions. In the case of hydroxypalmitic acid, the placement of the hydroxyl group adjacent to the carboxyl group (the 2-position) (B) allows the aliphatic C14 chain to freely interact with the stationary phase, whereas the placement of the hydroxyl group at the 16-position (A) interferes with this interaction and results in less retention on the column (Yeh and Ziemann, 2014). It is also worth noting the impact of these structural differences on the relative instrument sensitivities to A and B of about a factor of 3. Bi et al. observed that I-CIMS sensitivities can vary by 1–2 orders of magnitude for isomers of limonene oxidation products (Bi et al., 2021b).
Peak tailing was observed for most analytes and is more evident for those eluting in the latter half of the analysis. While total signal appeared to depend on the flow rate through the nebulizer bulb and VIA, peak shape did not (Fig. S2), indicating instead an upstream influence of the HPLC column or tubing. Comparing the results obtained here with those observed by Yeh and Ziemann (2014) and Schueneman et al. (2024), who used a similar system consisting of an HPLC coupled with TDPBMS and EESI-MS using a Collison atomizer, it is clear that the HPLC-VIA-CIMS provides better preservation of chromatographic peak shape between the HPLC and MS detection.
The thermal decomposition of compounds in the VIA has been previously reported as a limitation of this inlet (Häkkinen et al., 2023). If sufficient analyte separation is achieved by the HPLC, then all detectable thermal decomposition products can be attributed to the respective precursor ion based on retention time alignment. This feature would prove useful for the analysis of thermally labile compounds by HPLC-VIA-CIMS, such as multifunctional nitrates and peroxide-containing reaction products. Evidence of thermal decomposition was not observed for the carboxylic acid standards examined here, perhaps because of negligible decomposition or because the iodide reagent ion is not sensitive to the decomposition products. Full characterization of thermal decomposition is beyond the scope of this work, but it can be minimized by operating the VIA at lower temperatures or reducing residence time (Zhao et al., 2024).
To evaluate the linearity of the HPLC-VIA-CIMS response, seven-point calibration curves were generated with injection ranges of 0.2–3.0 nmol for all standards except for palmitic acid and stearic acid, for which a range of 2–30 nmol was used. The results are shown in Table 1. The system was found to have a linear response for all analytes (R2 > 0.97) across the tested range of mass loadings, illustrating the potential of the HPLC-VIA-CIMS system to be used for quantitation of analytes for which authentic standards can be purchased or synthesized. The calibration curves for each analyte are shown in Fig. S1.
3.2 α-pinene ozonolysis SOA
SOA formed by the ozonolysis of α-pinene was chosen for method evaluation with a complex OA as it has been well characterized and is known to contain carboxylic acid monomer and dimer products that are detected by I-CIMS with high sensitivity (Lopez-Hilfiker et al., 2014) and are often isobaric or differ by only two hydrogen atoms (Ma et al., 2007a; Zhao et al., 2023). Although other classes of compounds may co-elute with these (∼ 2000 compounds have been detected in this type of SOA; Heaton et al., 2009), they would need to have very similar chromatographic properties and be present in high abundance to compensate for the much lower detection sensitivity. The analysis of a 30 µg injection of the SOA by HPLC-VIA-CIMS identified numerous products, but for clarity a subset of these was selected here (and in the section below) based on relative contributions to the total I-CIMS signal (Figs. S3 and S4) and prevalence in the literature to illustrate the performance of the HPLC-VIA-CIMS system. Although a comprehensive analyte list was not compiled, the diversity of compounds detected by this system can be seen in the averaged mass spectra shown in Figs. S4 and S6. These spectra provide a qualitative view of the numerous other analytes detected in the mass range that was measured. It should be noted that the number of detectable compounds is more likely to be limited by injection mass rather than total collected aerosol mass.
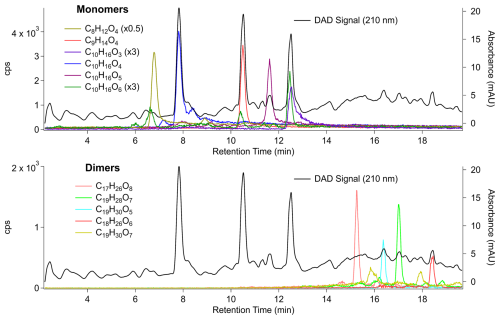
Figure 3HPLC-VIA-CIMS extracted ion chromatograms of a 30 µg injection of SOA generated by α-pinene ozonolysis. Monomer species (C8–10) are shown in the top chromatogram, and dimer species (C17–20) are shown in the bottom. Compounds were detected as I− adducts but are presented in their neutral form for clarity.
Chromatograms are shown in Fig. 3 and are divided into monomers (C8–10) and dimers (C17–19). The C8H12O4 (second peak, 9 min) and C10H16O3 (12.5 min) monomers were confirmed to be trans-norpinic acid and cis-pinonic acid, respectively, by comparing the retention times and peak shapes to those of authentic standards (Fig. S5). This suggests that these compounds and other carboxylic acids for which standards are available can be identified and quantified by HPLC-VIA-CIMS. A larger C8H12O4 peak was observed at a retention time of 7 min, which may be terpenylic acid as it is known to be produced by α-pinene ozonolysis in high yield (Claeys et al., 2009). And it is likely that cis-pinic acid (C9H14O4), a common product of α-pinene ozonolysis with a yield similar to that of cis-pinonic acid (Ma et al., 2007b), is observed at 10.5 min. This is supported by the difference in retention time between cis-pinonic acid (12.5 min) and C9H14O4 (10.5 min), as a more highly oxygenated compound with fewer carbon atoms would be expected to elute sooner from a reverse-phase HPLC column. A homologous series of monomer products with formulas of C10H16O4–6 eluting between 8 and 13 min was also observed. Of these, C10H16O4, corresponding to hydroxypinonic acid, and C10H16O6, an unidentified low-volatility product, were observed to have multiple isomers while only a single peak was detected for C10H16O5. It has been previously shown through ion mobility measurements that structural isomers of C10H16O4 and C10H16O6 exist among the products of α-pinene ozonolysis (Skyttä et al., 2022).
Several compounds suspected to be dimer products based on molecular formula (C17–19H26–30O5–8) were detected, and a subset is shown in Fig. 3. These have all been reported in other lab studies (Kenseth et al., 2023; Luo et al., 2024), and many have been observed in ambient OA (Kristensen et al., 2016). While isomeric structures of many dimer products in α-pinene ozonolysis SOA have been reported (Huang et al., 2018; Zhang et al., 2017), multiple chromatographic peaks were only observed here for C19H28O7 and C19H30O7. To the authors' knowledge, isomeric structures of these species have not been explicitly described in the literature, but hypothesized dimer formation mechanisms and the diversity of monomer precursors support their identification here. It is also possible that more than one isomer of C17H26O8, C19H28O7, and C19H30O5 exist in this sample but are too structurally similar to be resolved by the HPLC method that was used.
Absorbance at 210 nm was measured in addition to I-CIMS signal as monoterpene SOA has been shown to have broad absorption peaks at wavelengths below ∼ 300 nm (Bateman et al., 2011; Bones et al., 2010) and can thus be used to detect sufficiently absorbing species as they elute from the column. For reference, previous measurements on a series of standards determined molar absorptivities of about 4000, 200, 50, 3, and 3 M−1 cm−1 for a nitrate, alkenoic acid, carboxylic acid, alcohol, and ketone at 210 nm, respectively (Docherty and Ziemann, 2006). After retention time alignment with the I-CIMS time series, the DAD signal reveals the preservation of peak shape during nebulization and evaporation in the VIA, which is most clearly observed for the peaks at 8 min (C10H16O4), 10.5 min (C9H14O4), and 12.5 min (C10H16O5, C10H16O6) (Fig. 3, top). This is reasonable, since for an N2 flow rate of 3.5 L min−1 and a bulb volume of 300 mL, the residence time in the bulb is ∼ 5 s compared to a delay time between the DAD and CIMS signals of ∼ 2 s. This indicates that most of the aerosols are rapidly transmitted through the bulb to the VIA as well-defined packets with little mixing between them due to dispersion. This point is especially important for the identification of closely related structural isomers that may otherwise be attributed to peak splitting during the nebulization and evaporation of the HPLC eluent. Furthermore, for more strongly absorbing species such as nitrates, this approach may be used semi-quantitatively to determine relative abundances of closely related structures with similar molar absorptivities or, if authentic standards are available, absolute concentrations of these compounds.
3.3 Field-collected OA
The potential application of HPLC-VIA-CIMS to field-collected SOA was evaluated by analyzing an aerosol sample collected at the Denver, Colorado ASCENT site. Aerosol from this site was chosen as it was likely to contain multifunctional nitrates, which are easily detected by I-CIMS (He et al., 2024) and often occur as mixtures of isomers. The chromatogram generated from a 60 µg injection of this sample is shown in Fig. 4 and includes several compounds with molecular formulas that are consistent with multifunctional nitrates formed through monoterpene oxidation in an urban environment. The detection of these compounds is unsurprising given the prevalence of monoterpenes in the Denver area from biogenic emissions, personal care products, cannabis cultivation, and other sources (ASCENT, 2024; Gkatzelis et al., 2021; Wang et al., 2020). Although this analysis only highlights this subset of compounds, numerous other species were detected by HPLC-VIA-CIMS and are shown in Fig. S6 in the Supplement.
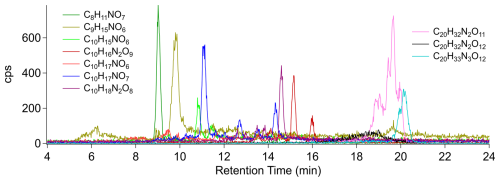
Figure 4HPLC-VIA-CIMS extracted ion chromatogram of a 60 µg injection of ambient SOA collected at the Denver, Colorado ASCENT site. The chemical formulas shown do not include I− but were detected as I− adducts.
Compounds suspected to be monoterpene oxidation products consisted of C8–9 species (assumed to be products of monomer decomposition reactions), C10 monomers, and C20 dimers. Specifically, C8H11NO7, C9H15NO6, C10H16N2O9, and C10H18N2O9 have been detected as products of the reaction of limonene with nitrate radicals (Faxon et al., 2018), whereas C10H15NO6, C10H17NO6, and C10H17NO7 are consistent with products formed through the oxidation of limonene and other monoterpenes by either nitrate radicals (Devault et al., 2022b) or by hydroxyl radicals in the presence of NOx (Devault et al., 2022a). It is worth noting the complex peak shapes of C10H17NO7 and C10H16N2O9 eluting between 11 and 16 min. Considering the ability of the nebulizer–VIA interface to transfer analytes from the HPLC to the I-CIMS with minimal peak distortion, it can be asserted that these peak shapes indicate the presence of multiple isomers and are not artifacts.
Three dimer species, C20H32N2O11, C20H32N2O12, and C20H32N3O12, were attributed to particle-phase accretion reactions of monomers formed through the reaction of monoterpenes with nitrate radicals (Devault et al., 2022b; Faxon et al., 2018; Takeuchi et al., 2022). Complex peak shapes were observed for C20H32N2O11 and C20H33N3O12, beginning at retention times of 18 and 19.5 min, respectively, which suggests the presence of multiple isomers. This is reasonable given the detection of monomer isomers and the possibility that these products were formed by several different monoterpene precursors. Multiple isomers of C20H33N3O12 were proposed by Takeuchi et al. (2022) in a study of nitrate radical oxidation of α-pinene/limonene mixtures, further supporting the assignment of these peaks.
The prevalence of organic nitrates and the absence of carboxylic acid products of monoterpene ozonolysis are likely due to the nighttime and daytime oxidation conditions under which the SOA was formed. As shown in Fig. S7, in the nighttime hours before sampling began, the O3, NO2, and NO concentrations were about 20, 10, and 0 ppb, respectively (Colorado Department of Public Health and Environment, 2025), due to the titration of NO to NO2 by reaction with O3. Although concentrations of NO3 radicals formed by the NO2 + O3 → NO3 + O2 reaction were not measured, values of < 0.5–100 ppt have been reported at field sites impacted by the Denver urban plume (Brown and Stutz, 2012). Given a rate constant ratio of about 7 × 104 for common monoterpenes such as limonene and α-pinene (Atkinson and Arey, 2003) and an assumed minimum [NO3] [O3] ratio of 1 ppt/20 ppb = 5 × 10−5, the minimum fraction of monoterpenes that would react with NO3 radicals would be ∼ 75 %. This indicates that the formation of organic nitrates by these reactions should dominate the fate of monoterpenes during this period. As NO concentrations increase at about 05:00 as rush hour begins, NO3 radicals will be removed by the NO3 + NO → 2NO2 reaction. Alkylperoxy radicals formed by reactions of monoterpenes with O3, and OH radicals formed by ozonolysis or by photolysis after sunrise at 07:00 (which will also photolyze NO3 radicals), will then react with NO to form organic nitrates and few carboxylic acids. The detection of C10HyNOx species in the OA samples is also consistent with measurements by Lee et al. (2016), who described a diurnal pattern of monoterpene-derived multifunctional alkyl nitrates, with concentrations peaking in the early morning hours, in the southeastern United States. Although their real-time measurements combined with modeling indicated lifetimes of 2–4 h, previous studies by Devault and Ziemann (2021) and Devault et al. (2022a, b) showed that organic nitrates formed from monoterpene oxidation reactions were stable for at least several days after filter sampling and extraction processes, similar to those used here. While it is possible that some of the CHNO species are not organic nitrates, our results, shown in Figs. 4 and S6, are consistent with the C10 organic nitrate distributions observed by Lee et al. (2016) during the early morning hours of their study period.
We have described and evaluated the coupling of an HPLC and an I-CIMS for the measurement of environmental chamber-generated and field-collected OA. The HPLC-VIA-CIMS achieved excellent separation of carboxylic acids, dicarboxylic acids, and hydroxycarboxylic acids, including a pair of structural isomers, while also having a linear response to analyte mass over an order of magnitude. The system also provided isomer-resolved detection of α-pinene ozonolysis products, including both monomer and dimer species that have been reported in previous studies of this reaction. Detection of these compounds by a DAD provided evidence for the high-fidelity transmission of chromatographic peaks from the HPLC to the I-CIMS. In future work, the DAD could be used to provide a semi-quantitative analysis of light-absorbing compounds for which standards are not available. This may prove particularly advantageous for chamber studies as HPLC-VIA-CIMS would enable direct measurements of species-specific particle-phase product yields, including yields of organic nitrates, which are important since they impact loss rates of gas-phase reactive nitrogen and therefore ozone formation. Although outside the scope of the present study, this apparatus could also be used to systematically evaluate possible artifacts incurred by filter sampling and other sample handling procedures used in offline analysis by comparing averaged mass spectra generated with HPLC-VIA-CIMS with those from online VIA-CIMS measurements. The analysis of field-collected OA by HPLC-VIA-CIMS produced similarly promising results, including the detection of several nitrogen-containing compounds attributed to monoterpene oxidation products. Even at low mass loadings (< 100 µg OA extract), excellent signal strength was achieved for numerous nitrate compounds, illustrating the potential of the HPLC-VIA-CIMS system to be used in studies of ambient aerosol composition, especially if detection limits can be lowered. This might be accomplished, for example, by improving the nebulizer design to increase transmission or by using other CIMS reagent ions that have higher sensitivity to compounds of interest. Finally, using HPLC-VIA-CIMS to characterize SOA enables the direct comparison with gas-phase species that are also detected using CIMS, simplifying investigations of semi-volatile compounds present in the atmosphere.
The data for this work are available upon request.
The supplement related to this article is available online at https://doi.org/10.5194/ar-3-557-2025-supplement.
AFS and PJZ conceptualized the project. AFS developed the nebulizer interface; performed the chamber experiment, sample preparation, and data analysis; generated the figures and tables; and wrote the paper. KHB reviewed and edited the paper. KEM provided guidance on laboratory experiments and also reviewed the paper. ERL organized the ambient SOA sample collection, and FM processed the sample. MRC and MWA provided the VIA, assisted with VIA operation, and reviewed the paper.
Manjula R. Canagaratna and Mitchell W. Alton are employees of Aerodyne Research Inc., which sells the VIA.
Publisher's note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors. Views expressed in the text are those of the authors and do not necessarily reflect the views of the publisher.
This research has been supported by the National Science Foundation, Directorate for Geosciences (grant no. AGS-1750447).
This paper was edited by Andreas Held and reviewed by Alexander Vogel and one anonymous referee.
Aljawhary, D., Lee, A. K. Y., and Abbatt, J. P. D.: High-resolution chemical ionization mass spectrometry (ToF-CIMS): application to study SOA composition and processing, Atmos. Meas. Tech., 6, 3211–3224, https://doi.org/10.5194/amt-6-3211-2013, 2013.
Alton, M., Canagaratna, M., Avery, A., Lambe, A., Kangasluoma, J., Ehn, M., Mickwitz, V., Zhao, J., and Worsnop, D.: Measurements of Atmospheric Aerosol with the Vaporization Inlet for Aerosols and the Filter Inlet for Gases and Aerosols on a Bipolar Multi-Reagent Chemical Ionization Mass Spectrometer, EGU General Assembly 2024, Vienna, Austria, 14–19 April 2024, EGU24-12470, https://doi.org/10.5194/egusphere-egu24-12470, 2024.
Apffel, A., Fischer, S., Goldberg, G., Goodley, P. C., and Kuhlmann, F. E.: Enhanced sensitivity for peptide mapping with electrospray liquid chromatography-mass spectrometry in the presence of signal suppression due to trifluoroacetic acid-containing mobile phases, J. Chromatogr. A., 712, 177–190, https://doi.org/10.1016/0021-9673(95)00175-M, 1995.
ASCENT: La Casa, https://ascent.research.gatech.edu/la-casa, last access: 9 December 2024.
Atkinson, R. and Arey, J.: Atmospheric degradation of volatile organic compounds, Chem. Rev., 103, 4605–4638, https://doi.org/10.1021/cr0206420, 2003.
Bateman, A. P., Nizkorodov, S. A., Laskin, J., and Laskin, A.: Photolytic processing of secondary organic aerosols dissolved in cloud droplets, Phys. Chem. Chem. Phys., 13, 12199–12212, https://doi.org/10.1039/c1cp20526a, 2011.
Bi, C., Krechmer, J. E., Frazier, G. O., Xu, W., Lambe, A. T., Claflin, M. S., Lerner, B. M., Jayne, J. T., Worsnop, D. R., Canagaratna, M. R., and Isaacman-VanWertz, G.: Coupling a gas chromatograph simultaneously to a flame ionization detector and chemical ionization mass spectrometer for isomer-resolved measurements of particle-phase organic compounds, Atmos. Meas. Tech., 14, 3895–3907, https://doi.org/10.5194/amt-14-3895-2021, 2021a.
Bi, C., Krechmer, J. E., Frazier, G. O., Xu, W., Lambe, A. T., Claflin, M. S., Lerner, B. M., Jayne, J. T., Worsnop, D. R., Canagaratna, M. R., and Isaacman-VanWertz, G.: Quantification of isomer-resolved iodide chemical ionization mass spectrometry sensitivity and uncertainty using a voltage-scanning approach, Atmos. Meas. Tech., 14, 6835–6850, https://doi.org/10.5194/amt-14-6835-2021, 2021b.
Bones, D. L., Henricksen, D. K., Mang, S. A., Gonsior, M., Bateman, A. P., Nguyen, T. B., Cooper, W. J., and Nizkorodov, S. A.: Appearance of strong absorbers and fluorophores in limonene-O3 secondary organic aerosol due to NH-mediated chemical aging over long time scales, J. Geophys. Res. Atmos., 115, D05203, https://doi.org/10.1029/2009JD012864, 2010.
Brown, S. S. and Stutz, J.: Nighttime radical observations and chemistry, Chem. Soc. Rev., 41, 6405–6447, https://doi.org/10.1039/c2cs35181a, 2012.
Cai, J., Daellenbach, K. R., Wu, C., Zheng, Y., Zheng, F., Du, W., Haslett, S. L., Chen, Q., Kulmala, M., and Mohr, C.: Characterization of offline analysis of particulate matter with FIGAERO-CIMS, Atmos. Meas. Tech., 16, 1147–1165, https://doi.org/10.5194/amt-16-1147-2023, 2023.
Canagaratna, M. R., Jayne, J. T., Jimenez, J. L., Allan, J. D., Alfarra, M. R., Zhang, Q., Onasch, T. B., Drewnick, F., Coe, H., Middlebrook, A., Delia, A., Williams, L. R., Trimborn, A. M., Northway, M. J., DeCarlo, P. F., Kolb, C. E., Davidovits, P., and Worsnop, D. R.: Chemical and microphysical characterization of ambient aerosols with the aerodyne aerosol mass spectrometer, Mass Spectrom. Rev., 26, 185–222, https://doi.org/10.1002/mas.20115, 2007.
Claeys, M., Iinuma, Y., Szmigielski, R., Surratt, J. D., Blockhuys, F., Van Alsenoy, C., Böge, O., Sierau, B., Gómez-González, Y., Vermeylen, R., Van Der Veken, P., Shahgholi, M., Chan, A. W. H., Herrmann, H., Seinfeld, J. H., and Maenhaut, W.: Terpenylic acid and related compounds from the oxidation of α-pinene: Implications for new particle formation and growth above forests, Environ. Sci. Technol., 43, 6976–6982, https://doi.org/10.1021/es9007596, 2009.
Colorado Department of Public Health and Environment: Air Quality Reports [data set], https://www.colorado.gov/airquality/report.aspx (last access: 21 March 2025), 2025.
Devault, M. P. and Ziemann, P. J.: Gas- and particle-phase products and their mechanisms of formation from the reaction of Δ-3-carene with NO3 radicals, J. Phys. Chem. A., 125, 10207–10222, https://doi.org/10.1021/acs.jpca.1c07763, 2021.
Devault, M. P., Ziola, A. C., and Ziemann, P. J.: Chemistry of secondary organic aerosol formation from reactions of monoterpenes with OH radicals in the presence of NOx, J. Phys. Chem. A, 126, 7719–7736, https://doi.org/10.1021/acs.jpca.2c04605, 2022a.
Devault, M. P., Ziola, A. C., and Ziemann, P. J.: Products and mechanisms of secondary organic aerosol formation from the NO3 radical-initiated oxidation of cyclic and acyclic monoterpenes, ACS Earth Space Chem., 6, 2076–2092, https://doi.org/10.1021/acsearthspacechem.2c00130, 2022b.
Docherty, K. S. and Ziemann, P. J.: Reaction of oleic acid particles with NO3 radicals: Products, mechanism, and implications for radical-initiated organic aerosol oxidation, J. Phys. Chem. A, 110, 3567–3577, https://doi.org/10.1021/jp0582383, 2006.
Docherty, K. S., Kumboonlert, K., Lee, I. J., and Ziemann, P. J.: Gas chromatography of trimethylsilyl derivatives of α-methoxyalkyl hydroperoxides formed in alkene-O3 reactions, J. Chromatogr. A., 1029, 205–215, https://doi.org/10.1016/j.chroma.2003.12.014, 2004.
Eichler, P., Müller, M., D'Anna, B., and Wisthaler, A.: A novel inlet system for online chemical analysis of semi-volatile submicron particulate matter, Atmos. Meas. Tech., 8, 1353–1360, https://doi.org/10.5194/amt-8-1353-2015, 2015.
Faxon, C., Hammes, J., Le Breton, M., Pathak, R. K., and Hallquist, M.: Characterization of organic nitrate constituents of secondary organic aerosol (SOA) from nitrate-radical-initiated oxidation of limonene using high-resolution chemical ionization mass spectrometry, Atmos. Chem. Phys., 18, 5467–5481, https://doi.org/10.5194/acp-18-5467-2018, 2018.
Finewax, Z., Jimenez, J. L., and Ziemann, P. J.: Development and application of a low-cost vaporizer for rapid, quantitative, in situ addition of organic gases and particles to an environmental chamber, Aerosol Sci. Tech., 54, 1567–1578, https://doi.org/10.1080/02786826.2020.1808186, 2020.
Gkatzelis, G. I., Coggon, M. M., McDonald, B. C., Peischl, J., Aikin, K. C., Gilman, J. B., Trainer, M., and Warneke, C.: Identifying volatile chemical product tracer compounds in U.S. cities, Environ. Sci. Technol., 55, 188-199, https://doi.org/10.1021/acs.est.0c05467, 2021.
Goldstein, A. H. and Galbally, I. E.: Known and unexplored organic constituents in the earth's atmosphere, Environ. Sci. Technol., 41, 1514–1521, https://doi.org/10.1021/es072476p, 2007.
Häkkinen, E., Zhao, J., Graeffe, F., Fauré, N., Krechmer, J. E., Worsnop, D., Timonen, H., Ehn, M., and Kangasluoma, J.: Online measurement of highly oxygenated compounds from organic aerosol, Atmos. Meas. Tech., 16, 1705–1721, https://doi.org/10.5194/amt-16-1705-2023, 2023.
He, S., Liu, Y., Song, M., Li, X., Lou, S., Ye, C., Liu, Y., Liu, Y., Ye, J., Lu, S., Zhou, W., Qiu, X., Zhu, T., and Zeng, L.: Empirical approach to quantifying sensitivity in different chemical ionization techniques for organonitrates and nitroaromatics constrained by ion-molecule reaction and transmission efficiency, Anal. Chem., 96, 16882–16890, https://doi.org/10.1021/acs.analchem.4c03751, 2024.
Hearn, J. D. and Smith, G. D.: A chemical ionization mass spectrometry method for the online analysis of organic aerosols, Anal. Chem., 76, 2820–2826, https://doi.org/10.1021/ac049948s, 2004.
Heaton, K. J., Sleighter, R. L., Hatcher, P. G., Hall IV, W. A., and Johnston, M. V.: Composition domains in monoterpene secondary organic aerosol, Environ. Sci. Technol., 43, 7797–7802, https://doi.org/10.1021/es901214p, 2009.
Hildmann, S. and Hoffmann, T.: Characterisation of atmospheric organic aerosols with one- and multidimensional liquid chromatography and mass spectrometry: State of the art and future perspectives, Trends Anal. Chem., 175, 117698, https://doi.org/10.1016/j.trac.2024.117698, 2024.
Hoffmann, T., Bandur, R., Marggraf, U., and Linscheid, M.: Molecular composition of organic aerosols formed in the α-pinene/O3 reaction: Implications for new particle formation processes, J. Geophys. Res. Atmos., 103, 25569–25578, https://doi.org/10.1029/98JD01816, 1998.
Huang, W., Saathoff, H., Pajunoja, A., Shen, X., Naumann, K.-H., Wagner, R., Virtanen, A., Leisner, T., and Mohr, C.: α-Pinene secondary organic aerosol at low temperature: chemical composition and implications for particle viscosity, Atmos. Chem. Phys., 18, 2883–2898, https://doi.org/10.5194/acp-18-2883-2018, 2018.
Kenseth, C. M., Hafeman, N. J., Rezgui, S. P., Chen, J., Huang, Y., Dalleska, N. F., Kjaergaard, H. G., Stoltz, B. M., Seinfeld, J. H., and Wennberg, P. O.: Particle-phase accretion forms dimer esters in pinene secondary organic aerosol, Science, 382, 787–792, https://doi.org/10.1126/science.adi0857, 2023.
Krechmer, J. E., Groessl, M., Zhang, X., Junninen, H., Massoli, P., Lambe, A. T., Kimmel, J. R., Cubison, M. J., Graf, S., Lin, Y.-H., Budisulistiorini, S. H., Zhang, H., Surratt, J. D., Knochenmuss, R., Jayne, J. T., Worsnop, D. R., Jimenez, J.-L., and Canagaratna, M. R.: Ion mobility spectrometry–mass spectrometry (IMS–MS) for on- and offline analysis of atmospheric gas and aerosol species, Atmos. Meas. Tech., 9, 3245–3262, https://doi.org/10.5194/amt-9-3245-2016, 2016.
Kristensen, K., Watne, Å. K., Hammes, J., Lutz, A., Petäjä, T., Hallquist, M., Bilde, M., and Glasius, M.: High-molecular weight dimer esters are major products in aerosols from α-pinene ozonolysis and the boreal forest, Environ. Sci. Technol. Lett., 3, 280–285, https://doi.org/10.1021/acs.estlett.6b00152, 2016.
Kroll, J. H. and Seinfeld, J. H.: Chemistry of secondary organic aerosol: Formation and evolution of low-volatility organics in the atmosphere, Atmos. Environ., 42, 3593–3624, https://doi.org/10.1016/j.atmosenv.2008.01.003, 2008.
Lee, B. H., Lopez-Hilfiker, F. D., Mohr, C., Kurtén, T., Worsnop, D. R., and Thornton, J. A.: An iodide-adduct high-resolution time-of-flight chemical-ionization mass spectrometer: Application to atmospheric inorganic and organic compounds, Environ. Sci. Technol., 48, 6309–6317, https://doi.org/10.1021/es500362a, 2014.
Lee, B. H., Mohr, C., Lopez-Hilfiker, F. D., and Thornton, J. A.: Highly functionalized organic nitrates in the southeast united states: contribution to secondary organic aerosol and reactive nitrogen budgets, P. Natl. Acad. Sci., 113, 1516–1521, https://doi.org/10.1073/pnas.1508108113, 2016.
Lobrutto, R., Jones, A., Kazakevich, Y. V., and Mcnair, H. M.: Effect of the eluent pH and acidic modifiers in high-performance liquid chromatography retention of basic analytes, J. Chromatogr. A, 913, 173–187, https://doi.org/10.1016/S0021-9673(00)01012-8, 2001.
Lopez-Hilfiker, F. D., Mohr, C., Ehn, M., Rubach, F., Kleist, E., Wildt, J., Mentel, Th. F., Lutz, A., Hallquist, M., Worsnop, D., and Thornton, J. A.: A novel method for online analysis of gas and particle composition: description and evaluation of a Filter Inlet for Gases and AEROsols (FIGAERO), Atmos. Meas. Tech., 7, 983–1001, https://doi.org/10.5194/amt-7-983-2014, 2014.
Luo, H., Guo, Y., Shen, H., Huang, D. D., Zhang, Y., and Zhao, D.: Effect of relative humidity on the molecular composition of secondary organic aerosols from α-pinene ozonolysis, Environ. Sci.: Atmos., 4, 519–530, https://doi.org/10.1039/d3ea00149k, 2024.
Ma, Y., Luciani, T., Porter, R. A., Russell, A. T., Johnson, D., and Marston, G.: Organic acid formation in the gas-phase ozonolysis of α-pinene, Phys. Chem. Chem. Phys., 9, 5084–5087, https://doi.org/10.1039/b709880d, 2007a.
Ma, Y., Willcox, T. R., Russell, A. T., and Marston, G.: Pinic and pinonic acid formation in the reaction of ozone with α-pinene, Chem. Commun., 1328–1330, https://doi.org/10.1039/b617130c, 2007b.
Mauderly, J. L. and Chow, J. C.: Health effects of organic aerosols, Inhal. Toxicol., 20, 257–288, https://doi.org/10.1080/08958370701866008, 2008.
Pöschl, U.: Atmospheric aerosols: Composition, transformation, climate and health effects, Angew. Chem. Int. Ed., 44, 7520–7540, https://doi.org/10.1002/anie.200501122, 2005.
Reinnig, M. C., Müller, L., Warnke, J., and Hoffmann, T.: Characterization of selected organic compound classes in secondary organic aerosol from biogenic VOCs by HPLC/MSn, Anal. Bioanal. Chem., 391, 171–182, https://doi.org/10.1007/s00216-008-1964-5, 2008.
Riva, M., Pospisilova, V., Frege, C., Perrier, S., Bansal, P., Jorga, S., Sturm, P., Thornton, J. A., Rohner, U., and Lopez-Hilfiker, F.: Evaluation of a reduced-pressure chemical ion reactor utilizing adduct ionization for the detection of gaseous organic and inorganic species, Atmos. Meas. Tech., 17, 5887–5901, https://doi.org/10.5194/amt-17-5887-2024, 2024.
Robinson, M. A., Roberts, J. M., Neuman, J. A., Jernigan, C. M., Xu, L., Coggon, M. M., Stockwell, C. E., Warneke, C., Peischl, J., Gilman, J. B., Lamplugh, A., Rollins, A. W., Zuraski, K., Rivera-Rios, J. C., Wang, Y., Ng, N. L., Liu, S., Brown, S. S., and Veres, P. R.: Online calibration of a chemical ionization mass spectrometer for multifunctional biogenic organic nitrates, ACS ES&T Air, 1, 1066–1083, https://doi.org/10.1021/acsestair.4c00056, 2024.
Ruiz-Jiménez, J., Hautala, S., Parshintsev, J., Laitinen, T., Hartonen, K., Petäjä, T., Kulmala, M., and Riekkola, M. L.: Aliphatic and aromatic amines in atmospheric aerosol particles: comparison of three ionization techniques in liquid chromatography-mass spectrometry and method development, Talanta, 97, 55–62, https://doi.org/10.1016/j.talanta.2012.03.062, 2012.
Schueneman, M. K., Day, D. A., Kim, D., Campuzano-Jost, P., Yun, S., DeVault, M. P., Ziola, A. C., Ziemann, P. J., and Jimenez, J. L.: A multi-instrumental approach for calibrating real-time mass spectrometers using high-performance liquid chromatography and positive matrix factorization, Aerosol Research, 2, 59–76, https://doi.org/10.5194/ar-2-59-2024, 2024.
Schwarzenbach, R.: High-performance liquid chromatography of carboxylic acids, J. Chromatogr. A, 251, 339–358, https://doi.org/10.1016/S0021-9673(00)87024-7, 1982.
Shrivastava, M., Cappa, C. D., Fan, J., Goldstein, A. H., Guenther, A. B., Jimenez, J. L., Kuang, C., Laskin, A., Martin, S. T., Ng, N. L., Petaja, T., Pierce, J. R., Rasch, P. J., Roldin, P., Seinfeld, J. H., Shilling, J., Smith, J. N., Thornton, J. A., Volkamer, R., Wang, J., Worsnop, D. R., Zaveri, R. A., Zelenyuk, A., and Zhang, Q.: Recent advances in understanding secondary organic aerosol: implications for global climate forcing, Rev. Geophys., 55, 509–559, https://doi.org/10.1002/2016RG000540, 2017.
Skyttä, A., Gao, J., Cai, R., Ehn, M., Ahonen, L. R., Kurten, T., Wang, Z., Rissanen, M. P., and Kangasluoma, J.: Isomer-resolved mobility-mass analysis of α-pinene ozonolysis products, J. Phys. Chem. A, 126, 5040–5049, https://doi.org/10.1021/acs.jpca.2c03366, 2022.
Takeuchi, M., Berkemeier, T., Eris, G., and Ng, N. L.: Non-linear effects of secondary organic aerosol formation and properties in multi-precursor systems, Nat. Commun., 13, https://doi.org/10.1038/s41467-022-35546-1, 2022.
Tobias, H. J. and Ziemann, P. J.: Compound identification in organic aerosols using temperature-programmed thermal desorption particle beam mass spectrometry, Anal. Chem., 71, 3428–3435, https://doi.org/10.1021/ac990056f, 1999.
Vasquez, K. T., Allen, H. M., Crounse, J. D., Praske, E., Xu, L., Noelscher, A. C., and Wennberg, P. O.: Low-pressure gas chromatography with chemical ionization mass spectrometry for quantification of multifunctional organic compounds in the atmosphere, Atmos. Meas. Tech., 11, 6815–6832, https://doi.org/10.5194/amt-11-6815-2018, 2018.
Voisin, D., Smith, J. N., Sakurai, H., McMurry, P. H., and Eisele, F. L.: Thermal desorption chemical ionization mass spectrometer for ultrafine particle chemical composition, Aerosol Sci. Tech., 37, 471–475, https://doi.org/10.1080/02786820300959, 2003.
Wang, C. T., Ashworth, K., Wiedinmyer, C., Ortega, J., Harley, P. C., Rasool, Q. Z., and Vizuete, W.: Ambient measurements of monoterpenes near cannabis cultivation facilities in Denver, Colorado, Atmos. Environ., 232, https://doi.org/10.1016/j.atmosenv.2020.117510, 2020.
Warneke, C., De Gouw, J. A., Kuster, W. C., Goldan, P. D., and Fall, R.: Validation of atmospheric VOC measurements by proton-transfer-reaction mass spectrometry using a gas-chromatographic preseparation method, Environ. Sci. Technol., 37, 2494–2501, https://doi.org/10.1021/es026266i, 2003.
Warscheid, B. and Hoffmann, T.: On-line measurements of α-pinene ozonolysis products using an atmospheric pressure chemical ionisation ion-trap mass spectrometer, Atmos. Environ., 35, 2927–2940, https://doi.org/10.1016/S1352-2310(00)00513-6, 2001.
Yeh, G. K. and Ziemann, P. J.: Identification and yields of 1,4-hydroxynitrates formed from the reactions of C8–C16 n-alkanes with OH radicals in the presence of NOx, J. Phys. Chem. A, 118, 8797–8806, https://doi.org/10.1021/jp505870d, 2014.
Yu, J., Flagan, R. C., and Seinfeld, J. H.: Identification of products containing -COOH, -OH, and -C=O in atmospheric oxidation of hydrocarbons, Environ. Sci. Technol., 32, 2357–2370, https://doi.org/10.1021/es980129x, 1998.
Yu, J., Griffin, R. J., Cocker, D. R., Flagan, R. C., Seinfeld, J. H., and Blanchard, P.: Observation of gaseous and particulate products of monoterpene oxidation in forest atmospheres, Geophys. Res. Lett., 26, 1145–1148, https://doi.org/10.1029/1999GL900169, 1999.
Zhang, X., Lambe, A. T., Upshur, M. A., Brooks, W. A., Gray Bé, A., Thomson, R. J., Geiger, F. M., Surratt, J. D., Zhang, Z., Gold, A., Graf, S., Cubison, M. J., Groessl, M., Jayne, J. T., Worsnop, D. R., and Canagaratna, M. R.: Highly oxygenated multifunctional compounds in α-pinene secondary organic aerosol, Environ. Sci. Technol., 51, 5932–5940, https://doi.org/10.1021/acs.est.6b06588, 2017.
Zhao, J., Häkkinen, E., Graeffe, F., Krechmer, J. E., Canagaratna, M. R., Worsnop, D. R., Kangasluoma, J., and Ehn, M.: A combined gas- and particle-phase analysis of highly oxygenated organic molecules (HOMs) from α-pinene ozonolysis, Atmos. Chem. Phys., 23, 3707–3730, https://doi.org/10.5194/acp-23-3707-2023, 2023.
Zhao, J., Mickwitz, V., Luo, Y., Häkkinen, E., Graeffe, F., Zhang, J., Timonen, H., Canagaratna, M., Krechmer, J. E., Zhang, Q., Kulmala, M., Kangasluoma, J., Worsnop, D., and Ehn, M.: Characterization of the Vaporization Inlet for Aerosols (VIA) for online measurements of particulate highly oxygenated organic molecules (HOMs), Atmos. Meas. Tech., 17, 1527–1543, https://doi.org/10.5194/amt-17-1527-2024, 2024.
Ziemann, P. J. and Atkinson, R.: Kinetics, products, and mechanisms of secondary organic aerosol formation, Chem. Soc. Rev., 41, 6582–6605, https://doi.org/10.1039/c2cs35122f, 2012.



