the Creative Commons Attribution 4.0 License.
the Creative Commons Attribution 4.0 License.
Linking biogenic high-temperature ice nucleating particles in Arctic soils and streams to their microbial producers
Julie K. Simonsen
Ada Pastor
Christof Pearce
Per Nørnberg
Lars Chresten Lund-Hansen
Kai Finster
Aerosols, including biological aerosols, exert a significant influence on cloud formation, influencing the global climate through their effects on radiative balance and precipitation. The Arctic region features persistent mixed-phase clouds, which are impacted by ice nucleating particles (INPs) that modulate the phase transitions within clouds, affecting their lifetime and impacting the region's climate. An increasing number of studies document that Arctic soils harbor numerous biogenic INPs (bioINPs), but these have yet to be linked to their microbial producers. In addition, the transfer of bioINPs from soils into freshwater and marine systems has not been quantified. This study aimed to address these open questions by analyzing soil and freshwater samples from northeast Greenland to determine the microbial composition along with the INP concentrations and size distributions. We found that soils contained between 3.19×104 and 1.55×106 INP g−1 soil, which was on the lower side of what has previously been reported for active-layer soils. The composition of INPs varied widely across locations and could have originated from bacterial and fungal sources. We detected Mortierella, a fungal genus known to produce ice nucleating proteins, at nearly all locations. Spearman correlations between soil taxa and INP concentrations pointed at lichenized fungi as a possible contributor to soil INP. Additionally, based on the INP size distribution, we suggest that soil INPs were bound to soil particles or microbial membranes at some locations, while other locations showed a variety of soluble INPs with different molecular sizes. In streams, INP concentrations were comparable to what has previously been measured in streams from temperate regions. Interestingly, stream INP concentrations showed a positive association with soil INP concentrations. The potential release and aerosolization of these bioINPs into the atmosphere – whether directly from the soil, from streams into which they are washed, or from the oceans where they might be transported – could impact cloud formation and precipitation patterns in the Arctic. This research contributes valuable knowledge to the understanding of microbial communities and the potential microbial producers of highly active bioINPs in Arctic soils and their connectivity with Arctic streams.
- Article
(5529 KB) - Full-text XML
-
Supplement
(1234 KB) - BibTeX
- EndNote
Clouds affect the global climate by regulating both surface precipitation and the atmosphere's radiative balance by absorbing, reflecting, and scattering radiation (Fan et al., 2016; Huang et al., 2021). Atmospheric aerosols affect cloud properties and precipitation through aerosol–cloud interactions, by acting as either cloud condensation nuclei to form cloud droplets or ice nucleating particles to form ice particles (Fan et al., 2016; Möhler et al., 2007). Among the various aerosol types, primary biological aerosol particles (PBAPs), were recognized ∼ 50 years ago for their high activity and ubiquitous distribution (Schnell and Vali, 1976; Vali et al., 1976). Recently, they have regained broader attention due to their potential to impact cloud formation and climate (Huang et al., 2021).
According to the Intergovernmental Panel on Climate Change (IPCC) report from 2023, the largest uncertainties in estimates of the Earth's changing energy budget are associated with cloud adjustments due to aerosols (IPCC, 2023). The Arctic region is especially sensitive to factors that trigger climate change due to Arctic amplification, which is the phenomenon when greenhouse-induced warming is enhanced and accelerated due to positive feedbacks between atmospheric processes, such as reduced convection and intrusion of humid air masses from the equatorial regions and reduced extent of sea ice, terrestrial ice, and snow cover (Holland and Bitz, 2003). Arctic amplification is responsible for a 2.5 °C higher surface air temperature increase in the Arctic compared to the global average (Serreze and Francis, 2006; Serreze and Barry, 2011). The predominant cloud type in the Arctic is mixed-phase clouds, which are composed of a mixture of supercooled droplets and ice particles (Fan et al., 2016). Arctic mixed-phase clouds are especially long-lived and can persist for several days (Morrison et al., 2011; Fan et al., 2016). They are important factors in controlling the Arctic climate, as they have a large impact on surface radiative fluxes and thus the energy balance (Fan et al., 2016; Morrison et al., 2011), which affects the ice melting rate (Carrió et al., 2005).
The number concentration of ice particles in mixed-phase clouds is vital because it influences the concentration of supercooled droplets and the size distribution of cloud particles and, hence, the optical properties of the cloud (DeMott et al., 2010). Ice formation in the atmosphere can occur in two ways, by either homogeneous or heterogeneous ice nucleation. Homogeneous freezing takes place in droplets of pure water that freeze at temperatures below −38 °C (Bigg, 1953). Heterogeneous freezing requires the presence of ice nucleating particles (INPs) such as dust, which facilitate the ice formation. In the presence of INPs, water droplets freeze at temperatures above −38 °C (Kanji et al., 2017). This is the case in mixed-phase clouds, where INPs greatly affect the lifetime and the radiative properties of the clouds (Hartmann et al., 2019).
INPs stem from various sources such as soil, vegetation, marine sea spray, and freshwater and can enter the atmosphere as, e.g., desert dust emissions, sea spray particles, anthropogenic emissions, and emissions from (micro)organisms (Huang et al., 2021).
Ice nucleation below −13 °C can be initiated by abiotic INPs, such as mineral particles or soot, or by incidental ice nucleation activity from biomolecules such as carbohydrates (Kinney et al., 2024). In contrast, proteinaceous INPs are the predominant type of INP active above −13 °C, which is shown by both laboratory studies and in situ measurements involving inactivation by heating (Murray et al., 2012; Cornwell et al., 2023; Kanji et al., 2017; Daily et al., 2022). Biological INPs include bacterial, fungal, and algal cells, as well as cell fragments and macromolecules, and have all been found in the Arctic atmosphere (Santl-Temkiv et al., 2022, 2019; Jensen et al., 2022; Kanji et al., 2017; Huang et al., 2021; Bigg, 1996; Bigg and Leck, 2001; Creamean et al., 2022; Stopelli et al., 2015, 2017; Hartmann et al., 2020, 2021; Ickes et al., 2020; Jayaweera and Flanagan, 1981; Porter et al., 2022).
Recent studies focused on the sources and abundance of atmospheric biogenic INPs (bioINPs) in the Arctic, with major contributions from terrestrial environments (Santl-Temkiv et al., 2019; Pereira Freitas et al., 2023; Sze et al., 2023). While soils in temperate regions are known to contain abundant and highly active bioINPs (Conen et al., 2011; Hill et al., 2016; O'Sullivan et al., 2016), there remains a significant knowledge gap when it comes to determining bioINP types, quantifying their numbers, and identifying microbial producers in the Arctic active-layer soils. In addition, their transfer to freshwater and marine systems is also poorly understood. In temperate regions, bioINPs have been associated with soil fungi affiliated with the genera Fusarium and Mortierella, which can produce ice nucleating proteins (INpros) (Fröhlich-Nowoisky et al., 2015; Pouleur et al., 1992). Highly active INPs have also been found in rivers in temperate regions (Larsen et al., 2017; Knackstedt et al., 2018; Moffett, 2016; Moffett et al., 2018). Similar to the emissions from seawater, aerosols can be formed from rivers and streams associated with turbulence due to high velocity and sloping terrain, rain splash, or obstacles interfering with the flow of water (Huang et al., 2021; Knackstedt et al., 2018; Raymond et al., 2012). Recent studies focusing on the Arctic found highly active INPs in the active layer and thawing permafrost soils and linked them to watersheds through wash-out and melting processes (Creamean et al., 2020; Barry et al., 2023a, b; Tobo et al., 2019). Once aerosolized, these soil INPs may impact regional clouds and thus the climate.
This study aims to investigate the following hypotheses: firstly, that specific microbial communities in terrestrial Arctic soils are a significant source of bioINPs and secondly, that there is a measurable link between soil and freshwater systems regarding the transport of these INPs in northeast Greenland. By analyzing the composition of the microbial communities in soils and quantifying INP concentrations and their size distribution in both soil and freshwater samples from adjacent streams, we aim to identify potential fungal and bacterial producers of INPs and quantify the outwash of INPs from soil into freshwater environments. While the direct flux quantifications from Arctic soils are beyond the scope of the study, we focus on understanding the transport and potential atmospheric impact of bioINPs from Arctic active-layer soils and freshwater systems. This approach will allow us to estimate the overall transport and cycling of INPs in Arctic ecosystems, providing data to reduce uncertainties concerning cloud microphysics, climate feedbacks, and cloud dynamics.
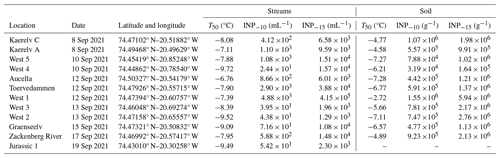
2.1 Sampling
The streams that were studied are located in Northeast Greenland National Park, near the Zackenberg Research Station (74°28′ N, 20°34′ W). Streamflow in the region is predominantly derived from melting snow and glaciers, with additional contributions varying by stream. For example, Kaerelv A, Kaerelv C, Graenseelv, and West 1 receive water from small, seasonal snow patches, while Aucella and Jurassic 1 are partly fed by larger ice aprons that are attached to mountainsides (Docherty et al., 2019; Hasholt and Hagedorn, 2000). This region has a polar tundra climate and is underlain by continuous permafrost with an active-layer thickness of 0.4–0.8 m (Christiansen et al., 2008; Hollesen et al., 2011). Geologically, the area is divided into crystalline complexes to the west and sedimentary successions to the east, with Quaternary sediments covering the valley floor and slopes. For a broader overview of the region's climate, geology, and vegetation, see Riis et al. (2023a). Surface soil and adjacent freshwater samples from streams were collected at 12 different locations in Zackenberg, Greenland (Fig. 1 and Table 1). The samples were collected in September 2021 over the course of 11 d. From each location, 14 mL of stream water and 14 mL of filtered stream water (0.22 µm polyethersulfone (PES)) were collected for INP quantification. From each location, approximately 3 g of surface soil was collected using a sterile spoon for INP quantification and microbial community profiling, making sure to collect the most abundant soil type without vegetation cover. No soil was collected at Jurassic 1 because the soil was frozen. All samples were stored at −20 °C. Freshwater and soil samples were also collected for chemical and physical characterization, which was reported by Riis et al. (2023a). Water chemistry parameters were measured, and snow cover percentage for each stream catchment was estimated by satellite images (Riis et al., 2023a).
Figure 1Map showing the locations of the 12 sampling sites in Zackenberg, Greenland. The map was created using QGIS. Geographical data are from Dataforsyningen (https://dataforsyningen.dk/, last access: 27 November 2024), with the Google Maps terrain as background (© Google Maps).
2.2 Quantification of INP
We used the micro-PINGUIN setup to determine INP concentrations as a function of temperature (Wieber et al., 2024a). The micro-PINGUIN consists of a cooling base, where a 384-well PCR plate is inserted, and a camera tower equipped with a thermal camera (FLIR A655sc) that measures the temperature in individual wells and allows for the determination of nucleation events. The tower was flushed with HEPA-filtered clean air for 5 min at a constant flow of 20 L min−1. Then, the samples were cooled at 1 K min−1 down to −30 °C while supplying a constant HEPA-filtered clean airflow between 5 and 10 L min−1 to keep the relative humidity low, avoiding condensation.
For each of the non-filtered freshwater samples, three dilutions were made (1:10, 1:100, and 1:1000). Samples filtered through a 0.22 µm PES (i.e., INP < 0.22 µm) were analyzed undiluted. To investigate different molecular size fractions of INPs, we further sequentially filtered selected samples of INP < 0.22 µm through 1000, 300, and 100 kDa filters (Vivaspin®, Sartorius). The freshwater samples, the dilutions, and the filtered freshwater samples were analyzed using the micro-PINGUIN setup. We analyzed 80 30 µL droplets per sample. Filtered Milli-Q (0.1 µm, PES) was used as a negative control. A custom-made software program (Ice Nucleation Controller) was used to obtain the frozen fractions.
The mean number of INPs per volume in the sample that are active at a given temperature can be calculated assuming that the locations of these INPs in the sample volume are statistically independent. Then, the probability of having a given number of droplets without INPs (that is, the fraction of no frozen droplets) is given by the binomial distribution, and the remaining fraction of droplets containing INPs (fraction of frozen droplets) leads to the following general equation:
where f is the frozen fraction (i.e., the fraction of frozen droplets), V is the droplet volume in each vial of the assay, and α is the number of droplets per sample (80 in this study).
Given that α≫1 in our study, the term is close to unity. Therefore Eq. (1) simplifies to Eq. (2):
This simplified form was used for the subsequent calculations in this study (Vali, 1971).
We prepared soil samples for ice nucleation analysis as previously described, with slight modifications (Conen and Yakutin, 2018). The soil samples were placed in a small Petri dish and freeze-dried overnight (Edwards Micro Modulyo Freeze Dryer). Freeze-drying was chosen instead of air-drying to minimize the potential for microbial activity during the drying process and to preserve the original composition of INPs. Prolonged air-drying could allow microbial activity while there is still sufficient liquid water available, which may alter INP concentrations due to production or degradation. The freeze-dried samples were kept in a desiccator to prevent rehydration and subsequently comminuted in a mortar by hand. Grinding was performed to break down aggregates formed during freeze-drying and ensured effective sieving. Given that the grinding was not performed with high intensity to specifically degrade cells or INPs, we do not expect significant degradation or loss of INPs within the samples. Samples were dry sieved with 125 and 63 µm sieves for 2 min using a vibratory sieve shaker (Analysette 3 PRO, Fritsch). The < 63 µm fraction was collected for analysis, as this size range represents the particles most likely to aerosolize (Fröhlich-Nowoisky et al., 2016). A sample of 100 mg of dry < 63 µm soil particles was weighed in an Eppendorf tube. For many samples, there was less than 100 mg of soil after sieving. Instead, all the sieved soil was added to the Eppendorf tube and the weight was noted. We added 1 mL filtered Milli-Q (0.22 µm PES) to the Eppendorf tube then vortexed it for 2 min and afterwards allowed it to settle for 10 min. We withdrew 0.5 mL from the top of the suspension and added it to a falcon tube with 9.5 mL of filtered Milli-Q (0.22 µm), creating a 1:20 dilution.
The soil suspensions were analyzed using the ice nucleation assay as described above. The number of INPs per gram that were active at a given temperature was calculated using Eq. (3):
where f is the frozen fraction, V is the droplet volume in each vial of the assay, Vs is the volume of the suspension, and m is the mass of soil in the suspension (Vali, 1971). Given the similar derivation of Eq. (3) to Eqs. (1) and (2), the assumption α≫1 applies here as well.
Freezing onset temperature was calculated as the temperature where 5 % of the wells for a given sample had frozen. T50 values were calculated as the temperature where 50 % of all wells for a given sample had frozen.
Additionally, we extracted the concentration of ice nucleation active (INA) particles at −10 °C (INP−10) and −15 °C (INP−15) per gram of soil or milliliter of stream water, for use in correlation analysis.
2.3 Soil total carbon and nitrogen measurements
Total carbon (TC) and total nitrogen (TN) were determined by combusting dry ball-mill-powdered soil in an elemental analyzer (Anca GSL2, Serco) coupled to an isotope ratio mass spectrometer (Hydra 20-22, Sercon). Aliquots between 10.06 and 31.83 mg soil were packed into tin cups and burned in the elemental analyzer. The content of carbon and nitrogen is given as mg C or N per kg dry soil.
2.4 Statistical analyses
The correlation between INP concentrations in water and soil were tested using Spearman's rank correlation analysis. The correlation between the INP concentrations, water chemistry, total carbon, and total nitrogen parameters were also tested using Spearman's rank correlation analysis. Significant differences in T50 values were computed by pooling all samples into different treatment groups and conducting a Kruskal–Wallis test. Significant differences between specific treatment groups were then investigated using a post hoc Wilcoxon rank sum test.
The correlation analyses were done in RStudio version 4.3.0 using Vegan (Dixon, 2003).
2.5 Grain size analysis
The particle size distributions were measured on a Sympatec Helos laser diffraction instrument equipped with a wet-dispersion input chamber. Samples were analyzed on R1 and R4 lenses, and measurements were subsequently combined to cover the range from 0.1 to 350 µm (Rasmussen, 2020). Only four samples contained enough particles for the analysis. The four samples that were analyzed were obtained from different geographical regions of the study area.
2.6 DNA extraction, quantitative polymerase chain reaction (qPCR), and amplicon sequencing
Amplicon sequencing targeting 16S rRNA, as well as the internal transcribed spacer (ITS) region, was used to determine the composition of the bacterial and fungal communities, respectively, while qPCR targeting the 16S rRNA gene was used to determine the quantity of bacteria in the soil.
DNA was extracted from 0.22 to 0.29 g of soil from samples where enough soil was present (Table S1) using the Power Soil Pro Kit (Qiagen, cat. no./ID 47016). Quantitative polymerase chain reaction (qPCR) using an MX3005p qPCR instrument (Agilent, Santa Clara, CA, United States) was performed to quantify the number of bacterial 16S rRNA gene copies. We targeted the partial 16S rRNA gene sequence using the universal primers Bac908F (5′-AAC TCA AAK GAA TTG ACG GG-3′) and Bac1075R (5′-CAC GAG CTG ACG ACA RCC-3′) (Ohkuma and Kudo, 1998). The qPCR mixture contained 2 µL template DNA, 1 µL of each primer (10 pmol µL−1), 2 µL bovine serum albumin (BSA) (10 ng µL−1), 10 µL LightCycler Mastermix (Roche), and 4 µL dH2O for a final volume of 20 µL. Samples were run in triplicate, and triplicate negative controls were run without the addition of template DNA. Samples were run at the following thermal conditions: 95 °C for 5 min, 45 cycles with denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, elongation at 72 °C for 15 s, followed by fluorescent acquisition at 80 °C for 5 s. A melting curve was produced using one cycle at 95 °C for 30 s, then ramping from 55 to 95 °C in 1 min before holding at 95 °C for 30 s.
The variable regions V3 and V4 of the 16S rRNA gene, which are commonly used for microbial community profiling due to their high variability among bacterial taxa, were amplified with the primers Bac341F (5′-CCT ACG GGN GGC WGC AG-3′) and Bac805R (5′-GAC TAC HVG GGT ATC TAA TCC-3′). The 16S rRNA gene amplification was performed according to a modified Illumina protocol. The PCR mixture contained 2 µL template DNA, 12.5 µL 2× KAPA HiFi HotStart polymerase (Kapa Biosystems, Inc., Wilmington, MA, United States), 0.5 µL 0.2 µM forward primer and 0.5 µL 0.2 µM reverse primer, and 9.5 µL dH2O for a final volume of 25 µL. The thermal cycling was run with an initial denaturation step at 95 °C for 3 min, 30 cycles with denaturation at 95 °C for 30 s, annealing at 55 °C for 30 s, elongation at 72 °C for 30 s, and a final elongation at 72 °C for 5 min. For the ITS library preparation we used the primers ITS3 (5′-GATGAAGAACGYAGYRAA-3′) and ITS4 (5′-CTBTTVCCKCTTCACTCG-3′) for amplification of the ITS2 region (Toju et al., 2012). The PCR mixture contained 10 µL template DNA, 12.5 µL 2× KAPA HiFi HotStart polymerase (Kapa Biosystems, Inc., Wilmington, MA, United States), 0.5 µL 0.2 µM forward primer and 0.5 µL 0.2 µM reverse primer, and 1.5 µL dH2O for a final volume of 25 µL. Thermal conditions were set as follows: an initial denaturation step at 95 °C for 3 min, followed by 22 cycles with denaturation at 95 °C for 30 s, annealing at 56 °C for 30 s, elongation at 72 °C for 30 s, and a final elongation at 72 °C for 5 min. The PCR products were cleaned using 30 µL AMPure XP magnetic beads for both the 16S and ITS amplicons. The second round of PCR was run with 2 µL of the amplified and cleaned PCR product for 10 cycles to incorporate overhang adapters and was run with the same conditions as the previous PCRs for the 16S and ITS thermal conditions. Products were cleaned, and the Nextera XT index primers were incorporated into a third PCR reaction, which was run for eight cycles following the previous conditions and with an annealing temperature of 55 °C. The PCR products were quantified using a Quant-iT™ dsDNA BR assay kit on a FLUOstar Omega fluorometric microplate reader (BMG LABTECH, Ortenberg, Germany), diluted and pooled together in equimolar ratios. The pool was quantified using the Quant-iT™ dsDNA BR assay kit on a Qubit fluorometer (Thermo Fisher Scientific, Waltham MA) and then sequenced on the Illumina MiSeq platform (Illumina, San Diego, CA), which produces two 300 bp long paired-end reads.
2.7 Bioinformatics analysis
Bioinformatics analyses were performed in RStudio 4.3.0. The 16S and ITS sequence reads were processed following a similar pipeline. For the 16S reads, primer and adapter sequences were trimmed from the raw reads using cutadapt 0.0.1 (Martin, 2011). The important differences in the ITS dataset are the identification and removal of primers from the reads and the verification of primer orientation and removal due to the variable length of the ITS amplicons. This is described in the DADA2 ITS Pipeline Workflow (1.8) (https://benjjneb.github.io/dada2/ITS_workflow.html, last access: 22 July 2024). Forward and reverse read quality were plotted using the plotQualityProfile function in DADA2 1.21.0 (Callahan et al., 2016). Based on the read quality, trimmings of 280 and 200 bp were set for the forward and reverse reads, respectively (for the 16S reads), using FilterAndTrim according to their quality (Callahan et al., 2016), whereas no trimming was done for the ITS sequences due to their variable lengths. Error models were built for the forward and reverse reads, followed by de-replication and clustering into amplicon sequence variants (ASVs) with DADA2 (Callahan et al., 2017). The denoised forward and reverse reads were merged using the function mergePairs, with default parameters that had a minimum overlap of 12 nucleotides, allowing 0 mismatches. Sequence tables were made with the function makeSequenceTable. ASVs shorter than 400 and longer than 430 nucleotides were removed from 16S, whereas all sequences were kept for the ITS dataset. Chimeric sequence removal was done using the removeBimeraDenovo function, and taxonomic assignment was accomplished using the naive Bayesian classifier against the SILVA ribosomal RNA gene database v138 (Quast et al., 2012) for the 16S rRNA sequences, while the UNITE database was used to classify the ITS sequences (Koljalg et al., 2005). ASVs mapped to mitochondria and chloroplasts were removed from the 16S dataset. Samples were decontaminated using the prevalence method (threshold = 0.1) from the Decontam package (Davis et al., 2018) using the DNA extraction negative control and the PCR negative control as the control group. Statistical tests and visualization of the data were performed with phyloseq (McMurdie and Holmes, 2013), Vegan (Dixon, 2003), and microeco (Liu et al., 2021).
3.1 Highly active INPs in northeast Greenlandic soils
By quantifying INP concentrations in soil samples (Fig. 2), we found that the onset of freezing was high (> −8 °C) at all locations (Fig. S1 in the Supplement). Thus, the freezing profiles are close to those of known proteinaceous INPs rather than carbohydrates (Kinney et al., 2024; Santl-Temkiv et al., 2022). The ice nucleation site density per gram of soil particles < 63 µm (Nm) as a function of temperature is depicted in Fig. 2 for all locations. INP−10 values varied by 2 orders of magnitude between locations, from 3.19×104 g−1 soil (West 4) up to 1.55×106 g−1 soil (West 1), which is also reflected in the INP−15 values (Table 1).
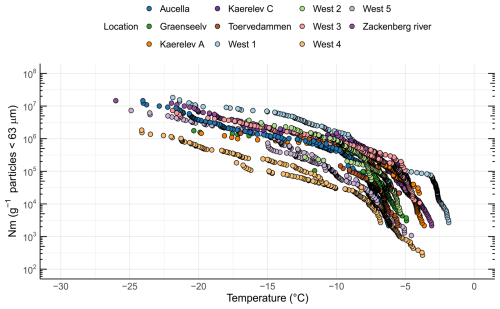
Figure 2INP concentrations (i.e., ice nucleation site density per gram of soil < 63 µm (Nm)) as a function of temperature in soil samples from 11 different locations in northeast Greenland.
Previous investigations showed that INPs in soils exhibited activity at temperatures above −15 °C and contain biological residues, in both temperate and Arctic regions (Conen et al., 2011; Huang et al., 2021). Several studies reported that INPs are more enriched in soils of colder climates compared to warmer climates (Creamean et al., 2020; Schnell and Vali, 1976; Tobo et al., 2019). A comparison of INP concentrations with previously published studies in the Arctic is shown in Fig. 3. Previous studies focused on similar latitudinal geographical regions while differing in longitude, ranging from Utqiagvik (USA) to Novosibirsk (Russia), central Yakutia (Russia), and Svalbard (Norway). Soil INP−10 concentrations ranged from 108 to 109 g−1 soil in Russia (Conen et al., 2011; Conen and Yakutin, 2018) to ∼ 108 g−1 soil in glacial outwash sediments in Svalbard (Tobo et al., 2019) and from > 108 g−1 down to 106 g−1 in permafrost soils from Utqiagvik (Barry et al., 2023b). In this study, we found the lowest INP−10 concentrations (ranging from ∼104 to ∼106 g−1). A possible explanation for the lower concentration could be the rather low carbon content of the soils measured in this study, with 9 out of 11 samples < 5 wt % TC, which is less than what Conen and Yakutin (2018) found in soils from central Yakutia but similar to the glacial outwash sediment that Tobo et al. (2019) investigated (Conen and Yakutin, 2018; Tobo et al., 2019). Higher carbon content in soils usually reflects a higher amount of biomass and microbial diversity present in the soil (Bastida et al., 2021). We also found significantly more biomass (16S rRNA copies) in soils with higher soil carbon content (R=0.8, p=0.0052). When relating the TC to the INP−10 concentrations in the soil, we found a non-significant negative correlation (, p=0.3270). Although carbon content has been proposed as an explanatory variable for INP−10 concentrations, reflecting higher microbial productivity, studies have not found a simple relationship between the two (Conen et al., 2011; Tobo et al., 2019). The higher INP concentrations reported by Barry et al. (2023b) may partly stem from their methodology, where active-layer soil was prepared by thawing and stirring without drying and sieving, directly suspending the bulk soil in water. This likely resulted in a broader representation of the particle size spectrum compared to our approach, where we used freeze-drying and comminuting in a mortar before differential settling focusing on isolating particles smaller than 63 µm. Similarly, Tobo et al. (2019), Conen et al. (2011), and Conen and Yakutin (2018) used air-drying to prepare soils before sieving through 90 and 63 µm meshes, respectively, to isolate finer fractions. They then further separated particles smaller than 5 µm using differential settling combined with filtration. The large variations in INP−10 between our study and results obtained by Tobo et al. (2019) and Conen et al. (2011) may be due to differences in microbial community composition and activity or in washout rates, which can be influenced by factors such as soil porosity and permeability (Wen et al., 2022). The compiled results indicate the presence of highly active INPs in Arctic soils, raising the potential for hydrological transport into river systems or oceans, as proposed by Creamean et al. (2020).
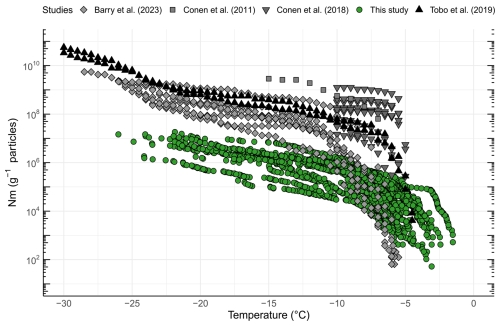
Figure 3Ice nucleation site density per gram of soil (Nm) as a function of temperature from five different studies. Light gray diamonds are derived from Barry et al. (2023b) (Utqiagvik – USA), gray squares are from Conen et al. (2011) (Novosibirsk – Russia), dark gray inverted triangles from Conen and Yakutin (2018) (central Yakutia – Russia), black triangles are from Tobo et al. (2019) (Svalbard – Norway), and green circles are from this study (Zackenberg – Greenland).
3.2 BioINPs in northeastern Greenlandic soils are diverse and potentially originate from different sources
To further characterize INPs within the Arctic soil, we used filtration analysis, as different microorganisms produce INpros of different molecular sizes, which can be either firmly bound to the cells or easily removed, resulting in soluble proteins (O'Sullivan et al., 2015; Santl-Temkiv et al., 2022). A similar approach has previously been used to study the origin of INP in environmental samples (Conen and Yakutin, 2018; Fröhlich-Nowoisky et al., 2015). A Kruskal–Wallis test indicated significant differences between the filtration treatments (p=0.0001) (Fig. 4). A post hoc Wilcoxon rank sum test showed a significant difference between the bulk sample (< 63 µm) and the 300–100 kDa fraction (p=0.0046), suggesting the predominance of INPs > 300 kDa. Subsequently, we analyzed the samples from individual locations to identify specific patterns.
The analysis reveals significant variations in the inferred composition of INPs within the soil across different locations (Fig. 4). At certain locations, such as West 4 and Aucella, there was a clear decrease in T50 activity following the filtration of samples through a 0.2 µm filter, which could be explained by the predominant association of INPs with microbial cells or with soil particles. O'Sullivan et al. (2016) demonstrated that INPs bind to clay particles smaller than 11 µm. They additionally found that typical topsoil pH (between 7.4 and 4.6) does not significantly affect this absorption. However, the presence of electrolytes such as CaCl2 and MgCl2, along with the contact time between clay particles and INPs, enhances absorption. Additionally, these clay particles were shown to aerosolize from the soil (O'Sullivan et al., 2016). Our study found that soil particle size distribution was relatively homogeneous across different locations, with approximately 15 %–20 % of soil particles being clay particles smaller than 5 µm (Fig. S2). This suggests that a portion of INPs would bind to these clay particles. Alternatively, the loss of activity may be explained by the presence of bacterial-membrane-bound INpros (Santl-Temkiv et al., 2022). Conversely, in samples from most locations, soluble INP was > 1000 kDa, which is in agreement with the formation of large bacterial INpro aggregates/oligomers within the 700 to 2000 kDa range, which are typically bound to membranes or membrane fragments (Schmid et al., 1997). In the sample from Kaerelv C, we observed predominant INPs in the size range between 300 and 1000 kDa, which is consistent with INpros produced by Fusarium acuminatum. The size of INpro molecules from F. acuminatum has been shown to be ∼ 5.3 kDa INPs, with aggregates reaching ∼ 700 kDa (Schwidetzky et al., 2023a). Finally, samples from Toervedammen, West 1, and Graenseelv contained predominant INPs with sizes between the 100 and 300 kDa filters. Studies by Fröhlich-Nowoisky et al. (2015) and Kunert et al. (2019) suggest that Mortierella sp. and Fusarium sp. produce INpro, which are between 300 and 100 kDa (Kunert et al., 2019). The gradual loss of INPs during filtration at the different locations suggests a mixture of different-sized INPs, likely originating from fungi and bacteria (Fig. 4). This interpretation is supported by the fact that fungal INPs are known to span a wide size range, including small-, < 100 kDa (e.g., 5 kDa), and medium-sized molecules (100–300 kDa and 300–1000 kDa), and can bind to clay particles, resulting in INPs > 1000 kDa and > 0.2 µm (Kunert et al., 2019; Schwidetzky et al., 2023b; O'Sullivan et al., 2015; Conen and Yakutin, 2018). The size distribution, combined with the observed solubility and ice nucleation activity, aligns with the characteristics of fungal INPs, further supporting their major contribution to the INP pool in these soils. Our findings somewhat align with Barry et al. (2023b), who demonstrated that the majority of INPs in soil were larger than 0.2 µm and were primarily of biological origin. Furthermore, their heat treatment experiments revealed that INPs in permafrost soil are predominantly heat-labile, further supporting their biological nature. Interestingly, Barry et al. (2023b) reported a limited presence of soluble INPs smaller than 0.2 µm in permafrost soil, suggesting a scarcity of such low-molecular-weight biological INPs in their study system. This contrasts with the observed presence of INPs spanning a large range of sizes in our samples, including < 100 kDa and aggregates > 1000 kDa (Fig. 4). Such discrepancies might reflect differences in the environmental conditions, microbial communities, or soil composition between their study sites and ours. Additionally, Barry et al. (2023a) highlighted that INP concentrations in permafrost soil are influenced by particle size and composition, observing that larger particles (> 10 µm) were significant contributors to INPs in younger permafrost samples. While their findings pertain to larger particle fractions, our study focused on the smaller clay-bound particles (< 5 µm) involved in transporting INPs in the atmosphere. While larger particles settle quickly after aerosolization, smaller clay-bound INPs are more prone to a longer atmospheric residence time after aerosolization (Meinander et al., 2022).
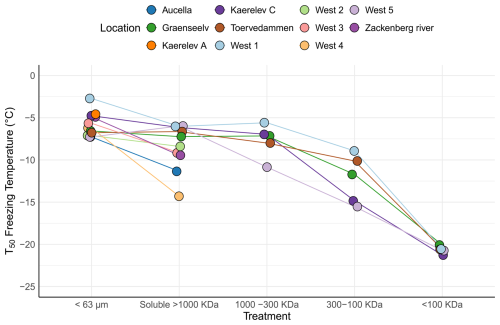
Figure 4Freezing activity (T50) for soil samples collected from 11 different locations in northeast Greenland. The samples underwent various filtration treatments. The < 63 µm category represents the untreated sample. The soluble > 1000 kDa category indicates samples that passed through a 0.2 µm filter but were retained on a 1000 kDa filter. The 1000–300 kDa category comprises samples that passed through the 1000 kDa filter but were retained on the 300 kDa filter. The 300–100 kDa category includes samples that passed through the 300 kDa filter but were retained on the 100 kDa filter. Finally, the < 100 kDa category encompasses samples that passed through the 100 kDa filter. Note that the samples from Aucella; Zackenberg; and West 2, 3, and 4 were only passed through the 0.2 µm filter, as they had already lost most of their high activity after this treatment.
The size distribution, combined with the observed solubility and ice nucleation activity, aligns with the characteristics of fungal INPs, further supporting their contribution to the INP pool in these soils. The INPs identified in Arctic soils have potential implications for atmospheric processes. Given that soil particles, including those bound to INPs, can become aerosolized through wind or other disturbances, these particles might contribute to atmospheric INP concentrations. Previous research has shown that soil dust and soil microorganisms can become a significant source of atmospheric aerosols in both temperate and Arctic regions (O'Sullivan et al., 2016; Tobo et al., 2019; Santl-Temkiv et al., 2018). Consequently, the high concentration of INPs in soil may enhance INP concentrations in the atmosphere, potentially impacting cloud formation and regional climate. Our findings align with studies indicating that INPs in soil environments can contribute to atmospheric INP levels. For instance, increased INP concentrations in Arctic soils might be linked to higher upward fluxes of atmospheric INP above these soils, similar to observations in other environments, e.g., arid soils, deserts, and agricultural soil (Kanji et al., 2017). This suggests that understanding soil INP sources is crucial for assessing their potential impact on atmospheric processes.
3.3 The link between the microbial community members and INPs
To link microbial community members to the INPs present in soils, we analyzed the bacterial and fungal communities in the soil samples. We found between 1.56×107 and 7.82×109 16S rRNA gene copies per gram of soil, which is within the same order of magnitude as has been found in similar soils (Ganzert et al., 2014; Santl-Temkiv et al., 2018). In total, we found that the bacterial community consisted of 6579 unique amplicon sequence variants (ASVs) (320–438 ASVs), which is on the lower side of the range found in soil bacterial communities across the Arctic region (Malard et al., 2019). The ASVs were dominated by members belonging to the phyla Proteobacteria (27.22±4.86 %) and Actinobacteria (18.60±9.46 %), while Planctomycetota, Bacteroiota, Acidobacteriota and Verrucomicrobiota each accounted for ∼ 8±4.66 % of the total community. Also, members of the following phyla were present in the samples: Cyanobacteria (5.3±7.12 %), Chloroflexi (5.29±5 %), Myxococcota (3.03±1.92 %), and Gemmatimonadota (2.32±1.08 %) (Fig. S3). Our results agree with the community data reported in Malard et al. (2019), showing consistency in the overall bacterial composition in Arctic soils.
By determining the sequences of the ITS region, we identified 2376 unique ASVs (63–234 ASVs) in total, which is comparable to the composition of fungal communities previously reported in Arctic soils (Varsadiya et al., 2021; Dziurzynski et al., 2023). The phylum Ascomycota was the most abundant phylum in all analyzed samples. It accounted for 60 %–70 % of all ASVs in 7 out of 10 locations (Fig. S4). A considerable fraction (23.6±19.8 %) of the ASVs could not be assigned to a specific phylum. The additional phyla were Basidiomycota (12.9±12.4 %), Chytridiomycota (2.4±2.6 %), and Mortierellomycota (1.2±2.4 %). We did not find any distinct geographical pattern for the composition of bacterial or fungal communities (Figs. 1, S3, and S4). Searching for known producers of INPs in the different soil samples, e.g., Pseudomonas, Erwinia, Pantoea, Xanthomonas, and Lysinibacillus, we only found 16S rRNA sequences related to Pseudomonas. These sequences were present at three locations at very low proportions (< 0.0006 %). Using a similar approach for fungi, we searched for the presence of ITS region sequences affiliated with Fusarium, Mortierella, Acremonium, Isaria, and Puccinia, which were previously reported to produce INPs (Pouleur et al., 1992; Fröhlich-Nowoisky et al., 2015; Huffman et al., 2013; Morris et al., 2013). Only sequences affiliated with the genera Acremonium and Mortierella were present in our dataset. Acremonium was only detected at one location, while Mortierella was present at most locations, albeit at a relatively low abundance (< 0.0025 %) (Fig. S5). Recent research has shown that INpros remain stable in permafrost soil for over 30 000 years and can retain their activity after thawing (Barry et al., 2023a; Creamean et al., 2020). Thus, INpros could have also been produced by past microbial communities and have accumulated in soils over time. Both habitat properties and plant cover were shown to affect abundance and diversity of Mortierella sp. in soils (Telagathoti et al., 2021; Mannisto et al., 2024; Shi et al., 2015). Arctic soils were typically found to contain a much higher fraction (on the order of 1 %–10 %) of Mortierella sp. compared to our study (Mannisto et al., 2024; Varsadiya et al., 2021). As Arctic terrestrial environments are undergoing dramatic changes, including changes in snow cover; soil development; and associated vegetation responses, e.g., the Arctic greening, soil microbial community composition is affected (Doetterl et al., 2021). Thus, past fungal communities at the sites we describe may have featured a higher abundance of Mortierella, which may have produced the observed INpros over time. Hence, as sequences affiliated with Mortierella were found at most locations, INA Mortierella sp. may have produced the highly potent INpros present in soils. This conclusion fits with filtration experiments that showed the presence of INpros at different soluble fractions at several locations, consistent with what has been shown for fungal INpros. While the particulate INpros present at other locations could be associated with bacterial INpros, this is not supported, given that bacterial genera known to produce INpros were not present consistently and that bacterial INpros have high temporal turnover rates (Watanabe et al., 1990). Thus, the particulate INpros are more likely to be associated with fungal INpros bound to clay particles.
An alternative explanation is that the INPs in the soils were produced by microorganisms not yet identified in the literature as those producing bioINPs. Over the past years it has become evident that the production of biogenic INA material is widespread throughout the tree of life, encompassing bacteria, algae, fungi, lichens, and pollen (Eufemio et al., 2023; Santl-Temkiv et al., 2022; Tesson and Santl-Temkiv, 2018; Adams et al., 2021; Gute et al., 2020). Based on these findings, a heterogeneous substrate like soil could potentially host many different taxa capable of producing INA material. Consequently, we used a bulk approach to link the high ice nucleation activity in the samples and the bacteria and fungi present in the soil. Using Spearman's rank correlation, we found that 14 bacterial and 3 fungal genera significantly positively correlated with the INP−10 and INP−15 concentrations (Fig. 5). Interestingly, none of the taxa correlating with INP−10 and INP−15 were related to known INP producers. These taxa could be previously unknown INpro producers. Their isolation from soil would be needed to establish whether they have the ability to produce INpro and unequivocally support the observed correlation. The bacteria were diverse and included phyla like Actinomycetota, Pseudomonadota, Verrucomicrobiota, Bacteroidota, Planctomycetota, and Cyanobacteria, highlighting the potential diversity of INP producers in soil ecosystems. While the Spearman rank correlations provided insights into potential associations between specific microbial genera and the concentration of INPs in soil samples, it is essential to acknowledge the limitations of this statistical approach. Correlations do not imply causation, and the observed relationships may be influenced by various confounding factors. Additionally, the Spearman rank correlation assesses monotonic relationships but may not capture nonlinear associations.
Fungal sequences that positively correlated with INP−10 and INP−15 concentrations were affiliated with the phylum Ascomycota. While this phylum is known to encompass many different lifestyles, only saprotrophic, pathogenic and lichenized fungi are known to produce INPs (Pouleur et al., 1992; Fröhlich-Nowoisky et al., 2015; Huffman et al., 2013; Morris et al., 2013). To understand the trophic mode and guilds of the potential fungal INP producers that we identified, we assigned these using the FungGuild database (Nguyen et al., 2016). We discovered that the predominant groups included fungi with different functions, comprising ectomycorrhizal fungi, wood saprotrophs, plant pathogens, and lichenized fungi (Fig. S6). This is in good agreement with Varsadiya et al. (2021), who also found that the ectomycorrhizal lifestyle dominated in Arctic top soil (Varsadiya et al., 2021). The fungal genus Atla, which shows a significant positive correlation with INP−10 and INP−15 number concentrations (see Fig. 5), was initially identified as a lichen by Savic and Tibell (Savić and Tibell, 2008). Lichens, known for their high ice nucleating activity, may contribute to the production of INPs in Arctic soil ecosystems. However, our knowledge of the structure of INPs produced by lichens is limited. Recent studies have shown that they are active at high temperatures (−3 °C), that they are not of bacterial origin (Moffett et al., 2015), and that they differ in their heat stability at 98 °C, with class A denaturing while class C retained activity that could suggest a combination of proteinaceous- and polysaccharide-based INPs (Eufemio et al., 2023).
The link between microbial communities and INPs has significant implications for atmospheric INP sources. Specific bacterial and fungal genera residing in soils are known to produce high-temperature proteinaceous INPs, making soil a significant source of atmospheric INPs emitted through wind erosion (Cornwell et al., 2023; Santl-Temkiv et al., 2022) and thus contributing to atmospheric INP concentrations (O'Sullivan et al., 2016; Bullard et al., 2016). Understanding the specific microbial taxa and the metabolic activities responsible for INP production in soils and streams is crucial to predict their contribution to atmospheric ice nucleation processes. This knowledge enables us to assess their impact on cloud formation, precipitation patterns, and regional climate dynamics (Keuschnig et al., 2023). Microbial community composition, including the abundance and activity of microbial strains that produce proteinaceous INPs, significantly changes as a response to environmental conditions, such as pH, organic matter content, nutrient availability, water activity, and Arctic greening (Malard et al., 2022; Wong et al., 2023). Thus, identifying key microbial INP producers and understanding their ecology and activity may have major implications for predicting the potential of different Arctic soils as reservoirs for INPs. This knowledge will feed into the potential to predict atmospheric INP concentrations and their overall impact on atmospheric processes and climate change.
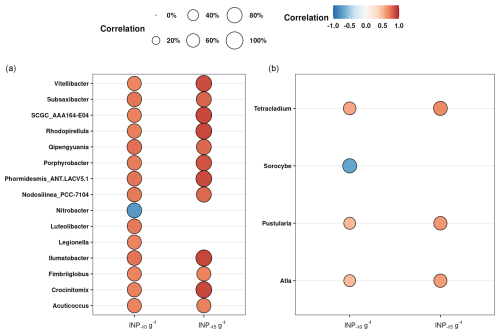
Figure 5Spearman's rank correlations between the concentrations of INP−10 and INP−15 g−1 smaller than 63 µm and the relative abundance of genera of bacteria (a) and fungi (b). The size of the bubbles represents the strength of the correlation coefficient, while the color indicates whether the correlation is positive (red) or negative (blue). We have excluded the correlations with INP−15 that are significant but have no significant correlation with INP−10, focusing only on the most intriguing taxa. To account for false positives and minimize false negatives, all p values have been adjusted using the Benjamin and Hochberg method (FDR).
3.4 Characterization of INPs in Greenlandic streams
In addition to characterizing soil INPs and their potential sources, we investigated the linkages between soil and freshwater INPs. The freezing onset was > −10 °C for all 12 water sampling locations (Fig. S7). The highest onset temperature was found in Aucella (−5.9 °C) and the lowest in West 4 (−9.1 °C). The high freezing temperatures indicate that the INPs are of biological origin and are consistent with the activity of proteinaceous INPs (Kanji et al., 2017). The ice nucleation site density per volume of freshwater (NV) as a function of temperature is shown in Fig. 6. The INP−10 concentration measured in our study (average of 1005 mL−1, range of 24–4880 mL−1) (Table 1) is much lower than those reported by Barry et al. (2023a) for Arctic thermokarst lakes (average of 34 300 mL−1, range of 1360–242 000 mL−1). One potential explanation for this difference is the higher soil INP concentrations reported in the Barry et al. (2023b) study compared to our measured soil INP concentrations. If soil is a major source of INPs for freshwater systems, as suggested by both studies, then lower soil INP concentrations in our sampling locations may directly contribute to the lower INP concentrations observed in Arctic streams relative to thermokarst lakes.
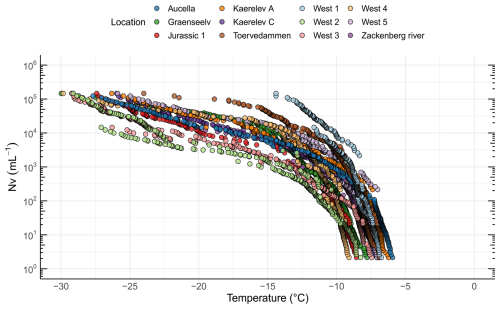
Figure 6Freezing activity and INP concentrations for freshwater samples from 12 different locations in northeast Greenland, showing the ice nucleation site density per volume (Nv) as a function of temperature.
Our results agree with what has been reported in previous studies, suggesting that INP−10 is omnipresent in freshwater systems (Larsen et al., 2017; Knackstedt et al., 2018; Moffett, 2016; Moffett et al., 2018). Since no Arctic rivers have been investigated prior to this study, we have turned towards comparisons with temperate regions that showed similar INP−10 concentrations as those reported here. Thus, a survey of a major river system in the USA (the Mississippi, Missouri, Platte, and Sweetwater rivers) found that the INP−10 number concentration ranged from 87 to 47 000 mL−1 (Moffett et al., 2018). A study of the River Gwaun in Wales found an INP−10 number concentration that ranged from 539 to 1570 mL−1 (Moffett, 2016). Additionally, INP−10 in the Maumee River (Ohio, USA) exceeded 10 000 mL−1 (Knackstedt et al., 2018), while INP−10 in the Rhine River (Switzerland) occasionally exceeded 1000 mL−1 (Larsen et al., 2017). The high concentrations of INP−10 in Arctic streams align with findings that several Arctic environments are rich in INPs, emphasizing their potential contribution as a regional source. However, despite these abundant local sources, aerosol INP concentrations in the Arctic atmosphere remain relatively low, possibly due to transport and deposition dynamics (Huang et al., 2021). The concentrations of INPs in stream water are greater than those typically found in marine systems. The average INP−10 found here was at least 7 times greater than that previously reported in Arctic marine systems, where INP−10 has been found to be between 10 and 100 mL−1 (Irish et al., 2017). A recent study demonstrated that terrestrial runoff in Greenland can significantly enhance INP concentrations in seawater in fjords (Wieber et al., 2024b). In addition, it has been estimated that the total freshwater discharge in Greenland increased from 136 Gt yr−1 in 1992 to 785 Gt yr−1 in 2012 (Mankoff et al., 2020). This suggests that freshwater is an increasing source of INPs in the Arctic oceans and that streams serve as a transport mechanism from soil into the ocean, or directly into the atmosphere from turbulence in the streams, with increased thawing due to warming (Rantanen et al., 2022).
To further characterize stream INPs, we used filtration experiments to compare with results obtained for the adjacent soil sampling sites. For most samples, T50 was higher for the filtered samples than for the non-filtered samples (Fig. 7). The same phenomenon has previously been described by Baloh et al. (2021) in river and pond samples in Obergurgl, Austria. They argue that a reasonable explanation is that some material in the samples counteracts ice nucleation, e.g., antifreeze proteins, and that these are removed by filtration, thereby increasing the ice nucleation activity in the filtrate (Baloh et al., 2021). This seems unlikely since antifreeze proteins are typically small and soluble; hence they would pass through the 0.22 µm filter (Davies, 2014; Lorv et al., 2014). Another possible explanation is that filtration through a 0.22 µm filter causes cell lysis. This could either release cell contents or break apart the membranes carrying INPs; thus the number of INPs would be increased in the filtrate. Bacterial and fungal cells can release INPs (Fröhlich-Nowoisky et al., 2015; Pummer et al., 2015; Phelps et al., 1986), and some INPs are thought to be associated with phytoplanktonic exudates (Wilson et al., 2015). The results from the filtration experiments compare well to other studies, suggesting that a majority of INPs in rivers and streams are soluble (Larsen et al., 2017; Moffett et al., 2018; Knackstedt et al., 2018). Statistical analysis using the Kruskal–Wallis test showed a significant difference among the treatments (). Post hoc Wilcoxon rank sum tests revealed a significant change from bulk to the 300–100 kDa category (p=0.0021). This was also observed in soil samples (Fig. 4), indicating that similar INPs are present in soil and streams, which further implies the possible transfer of INPs from soil into the streams.
The Aucella and Jurassic 1 streams showed the presence of INP > 1000 kDa, indicating the presence of bacterial INpros or fungal INpros bound to soil particles. Conversely, other streams contained mostly INP < 1000 kDa. Streams like Kaerelv C contained mostly INPs within the 300–1000 kDa range, and streams like Graenseelev had INPs between 100 and 300 kDa, both consistent with the fungal-like INpros seen in soil samples. In conclusion, INPs in the streams exhibit clear parallels with the soil samples in terms of their activity and size distributions. This finding highlights the potential for soil INPs to be transported into Arctic streams.
The INPs identified in Arctic streams provide additional insights into potential atmospheric sources of INPs. The moderate concentrations of INPs observed in the streams are higher than typical marine concentrations, suggesting that these waters might serve as a significant source of atmospheric INPs, especially given the observed variability in INP concentrations. Streams can act as conduits to transfer INPs from soil to the atmosphere, either through direct aerosolization caused by turbulence during high-flow conditions or by transporting these particles into the marine environment where they can subsequently aerosolize through sea spray (Huang et al., 2021; Knackstedt et al., 2018; Raymond et al., 2012; Wieber et al., 2024b). Our findings indicate that streams with high INP concentrations could contribute to atmospheric INP levels, similar to observations in other freshwater systems (Larsen et al., 2017; Knackstedt et al., 2018). The presence of high INP concentrations in Arctic streams suggests their potential role as local sources of atmospheric INPs, particularly in the context of Arctic warming and increased freshwater discharge (Mankoff et al., 2020). However, further studies are needed to explore the linkage between these sources and aerosolized INPs, as well as their broader implications for cloud formation and regional climate.
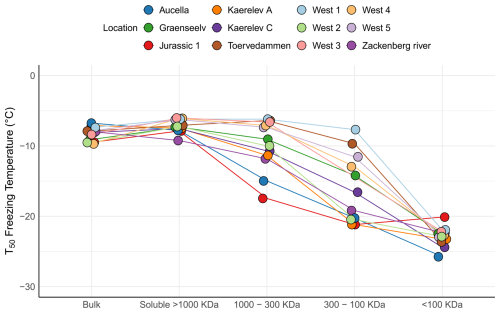
Figure 7Figure 7 illustrates the freezing activity at T50 for water samples collected from 12 different locations in northeast Greenland. The samples underwent various filtration treatments. The bulk category represents the untreated sample. The soluble > 1000 kDa category indicates samples that passed through a 0.2 µm filter but were retained on a 1000 kDa filter. The 1000–300 kDa category comprises samples that passed through the 1000 kDa filter but were retained on the 300 kDa filter. The 300–100 kDa category includes samples that passed through the 300 kDa filter but were retained on the 100 kDa filter. Finally, the < 100 kDa category encompasses samples that passed through the 100 kDa filter.
3.5 Sources of INPs in Greenlandic streams
Terrestrial runoff has previously been identified as a major source of INPs in temperate rivers and lakes (Larsen et al., 2017; Knackstedt et al., 2018) and in the Arctic ocean (Irish et al., 2017, 2019; Wieber et al., 2024b). Larsen et al. (2017) found that most highly active INPs in the river Rhine were transported to the freshwater system through surface or subsurface water flow. The primary sources of INPs were identified as plant surfaces, plant litter, and soil, with an implicit indication of bacterial and fungal sources. Due to a tight coupling of INPs to seasonal changes in river discharge, the watershed of the Maumee River was identified as the primary source of INPs to the river (Knackstedt et al., 2018).
If soil INPs are a major source of INPs in streams, we would expect to observe a positive correlation between soil and stream INP concentrations. Furthermore, in areas with steep terrain, less time is available for water to percolate through soil and accumulate INPs before entering the stream. Larger catchment areas, typically associated with longer streams, might also increase the potential for INP accumulation due to extended flow paths and surface interactions. Surprisingly, we did not observe significant correlations between INP−10 concentrations in streams and catchment area size or the slope of the terrain. Only a weak, non-significant correlation between soil and stream INP−10 concentrations was found (R=0.23, p=0.5031) (Fig. S8). These findings suggest that while soil may contribute INPs to streams, other factors such as local hydrology and dilution effects likely obscure a clear relationship. The onset freezing temperatures for the streams were in general lower compared to the adjacent soil samples, but these values are difficult to compare between such different systems, where the amount of input material evaluated with the droplet freezing assay can be vastly different (Figs. S1 and S7). However, in both soils and streams, INPs in the 300–100 kDa category were found at significantly lower concentrations than bulk INPs, which suggests that terrestrial runoff is a potential source of INPs in the streams.
Precipitation is unlikely to be a significant source of INPs in the streams studied, as INP concentrations in snow are typically much lower than those measured in the streams. Previous studies report INP−10 concentrations in snow ranging from as low as INP−10 mL−1 to approximately 8×101 INP−10 mL−1, which are significantly lower than the concentrations observed in stream water (Christner et al., 2008; Santl-Temkiv et al., 2019; Creamean et al., 2019; Brennan et al., 2020). This conclusion is also supported by Spearman's rank correlation analysis, which showed no correlation between percentage of watershed snow cover and INP−10 in the streams (p=0.8100) or soil (p=0.4630). This fits with previous studies of INPs in freshwater systems, where direct input to rivers from precipitation was found to be a minor contribution of INPs (Knackstedt et al., 2018; Moffett et al., 2018). Glacial outwash sediments were found to have a remarkably high nucleating ability (Tobo et al., 2019). Glacial outwash was shown to be the major freshwater runoff source in, e.g., Nuup Kangerlua, dominating rainfall and tundra, which would imply that streams mostly receive their water from this runoff (Oksman et al., 2022). Glacial outwash could therefore be an important source of INPs in streams.
Finally, INPs could be produced by microorganisms that are autochthonous to the streams. Only a few studies have investigated the INA organisms present in freshwater systems (Baloh et al., 2021; Benson et al., 2019), and different species of INA Pseudomonas have been found, which often grow in biofilms (Morris et al., 2007). Biofilms growing on stones, which could be potential sources of autochthonous INPs in streams, are complex aggregations of algae, bacteria, and fungi (Pastor et al., 2020; Battin et al., 2016). Morris et al. (2007) isolated 60–6000 P. syringae cells g−1 wet weight of biofilm from river biofilms at pristine settings characterized by low NO concentrations (Morris et al., 2007). A study on biofilm growth in the Graenseelv and Kaerelv streams found that biofilm accrual was largely driven by high NO concentrations in the stream (Pastor et al., 2020). In our study, we observed a weak, non-significant negative correlation between INP−10 in the streams and NO, total dissolved nitrogen (TDN), and dissolved inorganic nitrogen (DIN) concentrations (Fig. S8), which suggests that biofilms are not their predominant source. The size analysis of INPs in the streams (Fig. 7) is consistent with presence of predominantly fungal-type INpros at most locations, which are typically produced by soil fungi and could not be explained by the presence of INA bacteria in the stream biofilms. In addition, if a majority of INPs were produced in the streams, we would expect that changes in water chemistry, reflecting biogeochemical processes in the streams, would also correlate with changes in INP concentration. Aside from the NO3, TDN, and DIN, we observed no other correlations between INP−10 and chemical parameters using Spearman's rank correlation (Fig. S8). Overall, we found no evidence for autochthonous production of INPs in the streams based on the parameters that we recorded. To better understand the microbial transfer dynamics between soils and streams, future studies should include parallel analyses of both soil and stream microbial communities. This would enable a more detailed assessment of microbial transfer, particularly for ice nucleating taxa, which is crucial to understand the links between these two environments.
This study presents a detailed analysis of soil and freshwater INPs in high-Arctic Greenland, offering critical insights into their sources and diversity. The findings for the first time describe parallel measurements of INP concentrations in Arctic soil and stream systems and incorporate microbial community analysis, providing direct connections between microbial taxa and their respective INP contributions, which opens the necessity for more studies investigating these environments. We found that soil contained INPs that induced freezing at high temperatures, i.e., generally above −8 °C. The INP−10 concentrations varied by 2 orders of magnitude between locations, from 3.19×104 g−1 to 1.55×106 g−1. Additionally, using filtration through a series of filters with decreasing cut-offs, we found that soil INPs at some locations were associated with soil particles or microbial membranes, while at other locations they were present in the soluble fraction, likely excreted by or detached from microbial membranes. We found that Mortierella, a potential producer of INPs, was present across most locations and that this taxon could be responsible for the production of these bioINPs. We took a novel approach using Spearman's rank correlations between taxa relative abundance and INP concentration, which provided new insights into potential producers of the warm-temperature INPs. Based on these correlations, we hypothesize that a diverse and hitherto-overlooked number of bacterial genera and fungi could produce these warm-temperature INPs. To test this hypothesis, the identified taxa need to be isolated from Arctic soil. Alternatively, representatives of the respective taxa that are stored in culture collections could be tested for their ice nucleation activity.
In streams, INP concentrations were similar to those observed in temperate region rivers, such as the Mississippi and Gwaun rivers. However, these concentrations were lower than those reported in other Arctic freshwater systems like thermokarst lakes. These concentrations demonstrated a positive but not significant correlation with INP concentrations in soil, which indicates that INPs are transported from soil into adjacent streams but are not the sole source of stream INPs.
Our findings support the previously posed hypothesis that Arctic soil acts as an INP reservoir for streams. These INPs were able to aerosolize directly from the soil or the streams or can be transported further into the ocean, getting airborne through sea spray. In this way, the highly active INPs could impact cloud formation and climate, implying that bioINPs from soils and streams play a significant yet complex role in the Arctic climate system. Our findings underscore the importance of understanding the transport and potential atmospheric impact of bioINPs. While the direct quantification of aerosolization was beyond the scope of this study, future research should focus on deciphering the contributions from various sources, such as active-layer soil, runoff, and marine emissions, combining the approaches used in this study with those employed in studies like Barry et al. (2023a, b) to fully elucidate their roles in cloud formation and climate processes. As permafrost thaws and glaciers recede at an alarming rate, it has become increasingly important to understand the potential impact of these changes on INP concentrations in the Arctic. Understanding the origins and prevalence of INPs in this region, especially in the context of a warming climate, holds significance for accurate climate predictions. Thus, this research is an important contribution to understanding the dynamics of microbial communities and identifying the key contributors responsible for producing highly active INPs within Arctic soil microbial communities.
Ice nucleation data and soil geological data are available upon request. Stream biogeochemical data and snow cover data are available at https://doi.org/10.1594/PANGAEA.963212 (Riis et al., 2023b). Sequence data obtained from this project are available at the European Nucleotide Archive under the accession number PRJNA1137255.
The supplement related to this article is available online at https://doi.org/10.5194/ar-3-81-2025-supplement.
TŠ-T, KF, and AP designed the research project. TŠ-T, LCL-H, and LZJ supervised sample collection. AP collected all samples. PN supervised the preparation of soil samples. JKS and LZJ prepared all samples and performed all ice nucleation measurements. LZJ performed the bioinformatics analysis. CP conducted the grain size distribution analyses. LZJ, JKS, KF, and TŠ-T wrote the paper, with contributions from all other coauthors.
The contact author has declared that none of the authors has any competing interests.
Publisher’s note: Copernicus Publications remains neutral with regard to jurisdictional claims made in the text, published maps, institutional affiliations, or any other geographical representation in this paper. While Copernicus Publications makes every effort to include appropriate place names, the final responsibility lies with the authors.
The authors are grateful to Egon R. Fransen for logistical support and to Zackenberg logistics for assistance at the Zackenberg Research Station. We thank Britta Poulsen for excellent technical assistance with DNA extractions and sequencing, Gunnar Rasmussen for help with TC and TN measurements, and Corina Wieber for assistance with the ice nucleation setup. We also appreciate the support of Núria Catalán and Cecilie M. H. Holmboe with sample collection.
This work was supported by the Novo Nordisk Foundation (NNF19OC0056963), the Villum Foundation (23175, 28351, and 37435), the Danish National Research Foundation (DNRF106 to the Stellar Astrophysics Centre, Aarhus University), the Danish Agency for Higher Education and Science (1113-00025B), and the Carlsberg Foundation (CF21-0630 and CF24-1911). The project was further developed within the framework of FACE-IT, which has received funding from the European Union's Horizon 2020 research and innovation program under grant agreement no. 869154.
This paper was edited by Jose Castillo and reviewed by three anonymous referees.
Adams, M. P., Atanasova, N. S., Sofieva, S., Ravantti, J., Heikkinen, A., Brasseur, Z., Duplissy, J., Bamford, D. H., and Murray, B. J.: Ice nucleation by viruses and their potential for cloud glaciation, Biogeosciences, 18, 4431–4444, https://doi.org/10.5194/bg-18-4431-2021, 2021.
Baloh, P., Hanlon, R., Anderson, C., Dolan, E., Pacholik, G., Stinglmayr, D., Burkart, J., Felgitsch, L., Schmale, D. G., and Grothe, H.: Seasonal ice nucleation activity of water samples from alpine rivers and lakes in Obergurgl, Austria, Sci. Total Environ., 800, 149442, https://doi.org/10.1016/j.scitotenv.2021.149442, 2021.
Barry, K. R., Hill, T. C. J., Moore, K. A., Douglas, T. A., Kreidenweis, S. M., DeMott, P. J., and Creamean, J. M.: Persistence and Potential Atmospheric Ramifications of Ice-Nucleating Particles Released from Thawing Permafrost, Environ. Sci. Technol., 57, 3505–3515, https://doi.org/10.1021/acs.est.2c06530, 2023a.
Barry, K. R., Hill, T. C. J., Nieto-Caballero, M., Douglas, T. A., Kreidenweis, S. M., DeMott, P. J., and Creamean, J. M.: Active thermokarst regions contain rich sources of ice-nucleating particles, Atmos. Chem. Phys., 23, 15783–15793, https://doi.org/10.5194/acp-23-15783-2023, 2023b.
Bastida, F., Eldridge, D. J., Garcia, C., Kenny Png, G., Bardgett, R. D., and Delgado-Baquerizo, M.: Soil microbial diversity-biomass relationships are driven by soil carbon content across global biomes, ISME J., 15, 2081–2091, https://doi.org/10.1038/s41396-021-00906-0, 2021.
Battin, T. J., Besemer, K., Bengtsson, M. M., Romani, A. M., and Packmann, A. I.: The ecology and biogeochemistry of stream biofilms, Nat Rev Microbiol, 14, 251–263, https://doi.org/10.1038/nrmicro.2016.15, 2016.
Benson, J., Hanlon, R., Seifried, T., Baloh, P., Powers, C., Grothe, H., and Schmale, D.: Microorganisms Collected from the Surface of Freshwater Lakes Using a Drone Water Sampling System (DOWSE), Water, 11, 157, https://doi.org/10.3390/w11010157, 2019.
Bigg, E. K.: The formation of atmospheric ice crystals by the freezing of droplets, Q. J. Roy. Meteor. Soc., 79, 510–519, https://doi.org/10.1002/qj.49707934207, 1953.
Bigg, E. K.: Ice forming nuclei in the high Arctic, Tellus B, 48, 223–233, https://doi.org/10.1034/j.1600-0889.1996.t01-1-00007.x, 1996.
Bigg, E. K. and Leck, C.: Cloud-active particles over the central Arctic Ocean, J. Geophys. Res.-Atmos., 106, 32155–32166, https://doi.org/10.1029/1999jd901152, 2001.
Brennan, K. P., David, R. O., and Borduas-Dedekind, N.: Spatial and temporal variability in the ice-nucleating ability of alpine snowmelt and extension to frozen cloud fraction, Atmos. Chem. Phys., 20, 163–180, https://doi.org/10.5194/acp-20-163-2020, 2020.
Bullard, J. E., Baddock, M., Bradwell, T., Crusius, J., Darlington, E., Gaiero, D., Gassó, S., Gisladottir, G., Hodgkins, R., McCulloch, R., McKenna-Neuman, C., Mockford, T., Stewart, H., and Thorsteinsson, T.: High-latitude dust in the Earth system, Rev. Geophys., 54, 447–485, https://doi.org/10.1002/2016rg000518, 2016.
Callahan, B. J., McMurdie, P. J., Rosen, M. J., Han, A. W., Johnson, A. J., and Holmes, S. P.: DADA2: High-resolution sample inference from Illumina amplicon data, Nat. Methods, 13, 581–583, https://doi.org/10.1038/nmeth.3869, 2016.
Callahan, B. J., McMurdie, P. J., and Holmes, S. P.: Exact sequence variants should replace operational taxonomic units in marker-gene data analysis, ISME J., 11, 2639–2643, https://doi.org/10.1038/ismej.2017.119, 2017.
Carrió, G. G., Jiang, H., and Cotton, W. R.: Impact of Aerosol Intrusions on Arctic Boundary Layer Clouds. Part II: Sea Ice Melting Rates, J. Atmos. Sci., 62, 3094–3105, https://doi.org/10.1175/JAS3558.1, 2005.
Christiansen, H. H., Sigsgaard, C., Humlum, O., Rasch, M., and Hansen, B. U.: Permafrost and Periglacial Geomorphology at Zackenberg, in: High-Arctic Ecosystem Dynamics in a Changing Climate, Adv. Ecol. Res., 40, 151–174, https://doi.org/10.1016/s0065-2504(07)00007-4, 2008.
Christner, B. C., Morris, C. E., Foreman, C. M., Cai, R., and Sands, D. C.: Ubiquity of biological ice nucleators in snowfall, Science, 319, 1214, https://doi.org/10.1126/science.1149757, 2008.
Conen, F. and Yakutin, M. V.: Soils rich in biological ice-nucleating particles abound in ice-nucleating macromolecules likely produced by fungi, Biogeosciences, 15, 4381–4385, https://doi.org/10.5194/bg-15-4381-2018, 2018.
Conen, F., Morris, C. E., Leifeld, J., Yakutin, M. V., and Alewell, C.: Biological residues define the ice nucleation properties of soil dust, Atmos. Chem. Phys., 11, 9643–9648, https://doi.org/10.5194/acp-11-9643-2011, 2011.
Cornwell, G. C., McCluskey, C. S., Hill, T. C. J., Levin, E. T., Rothfuss, N. E., Tai, S.-L., Petters, M. D., DeMott, P. J., Kreidenweis, S., Prather, K. A., and Burrows, S. M.: Bioaerosols are the dominant source of warm-temperature immersion-mode INPs and drive uncertainties in INP predictability, Sci. Adv., 9, eadg3715, https://doi.org/10.1126/sciadv.adg3715, 2023.
Creamean, J. M., Mignani, C., Bukowiecki, N., and Conen, F.: Using freezing spectra characteristics to identify ice-nucleating particle populations during the winter in the Alps, Atmos. Chem. Phys., 19, 8123–8140, https://doi.org/10.5194/acp-19-8123-2019, 2019.
Creamean, J. M., Hill, T. C. J., DeMott, P. J., Uetake, J., Kreidenweis, S., and Douglas, T. A.: Thawing permafrost: an overlooked source of seeds for Arctic cloud formation, Environ. Res. Lett., 15, 084022, https://doi.org/10.1088/1748-9326/ab87d3, 2020.
Creamean, J. M., Barry, K., Hill, T. C. J., Hume, C., DeMott, P. J., Shupe, M. D., Dahlke, S., Willmes, S., Schmale, J., Beck, I., Hoppe, C. J. M., Fong, A., Chamberlain, E., Bowman, J., Scharien, R., and Persson, O.: Annual cycle observations of aerosols capable of ice formation in central Arctic clouds, Nat. Commun., 13, 3537, https://doi.org/10.1038/s41467-022-31182-x, 2022.
Daily, M. I., Tarn, M. D., Whale, T. F., and Murray, B. J.: An evaluation of the heat test for the ice-nucleating ability of minerals and biological material, Atmos. Meas. Tech., 15, 2635–2665, https://doi.org/10.5194/amt-15-2635-2022, 2022.
Davies, P. L.: Ice-binding proteins: a remarkable diversity of structures for stopping and starting ice growth, Trends Biochem. Sci., 39, 548–555, https://doi.org/10.1016/j.tibs.2014.09.005, 2014.
Davis, N. M., Proctor, D. M., Holmes, S. P., Relman, D. A., and Callahan, B. J.: Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data, Microbiome, 6, 226, https://doi.org/10.1186/s40168-018-0605-2, 2018.
DeMott, P. J., Prenni, A. J., Liu, X., Kreidenweis, S. M., Petters, M. D., Twohy, C. H., Richardson, M. S., Eidhammer, T., and Rogers, D. C.: Predicting global atmospheric ice nuclei distributions and their impacts on climate, P. Natl. Acad. Sci. USA, 107, 11217–11222, https://doi.org/10.1073/pnas.0910818107, 2010.
Dixon, P.: VEGAN, a package of R functions for community ecology, J. Veg. Sci., 14, 927–930, https://doi.org/10.1111/j.1654-1103.2003.tb02228.x, 2003.
Docherty, C. L., Dugdale, S. J., Milner, A. M., Abermann, J., Lund, M., and Hannah, D. M.: Arctic river temperature dynamics in a changing climate, River Re. Appl., 35, 1212–1227, https://doi.org/10.1002/rra.3537, 2019.
Doetterl, S., Alexander, J., Fior, S., Frossard, A., Magnabosco, C., van de Broek, M., and Westergaard, K. B.: Will accelerated soil development be a driver of Arctic Greening in the late 21st century?#, J. Plant Nutr. Soil Sc., 185, 19–23, https://doi.org/10.1002/jpln.202100334, 2021.
Dziurzynski, M., Gorecki, A., Pawlowska, J., Istel, L., Decewicz, P., Golec, P., Styczynski, M., Poszytek, K., Rokowska, A., Gorniak, D., and Dziewit, L.: Revealing the diversity of bacteria and fungi in the active layer of permafrost at Spitsbergen island (Arctic) – Combining classical microbiology and metabarcoding for ecological and bioprospecting exploration, Sci. Total Environ., 856, 159072, https://doi.org/10.1016/j.scitotenv.2022.159072, 2023.
Eufemio, R. J., de Almeida Ribeiro, I., Sformo, T. L., Laursen, G. A., Molinero, V., Fröhlich-Nowoisky, J., Bonn, M., and Meister, K.: Lichen species across Alaska produce highly active and stable ice nucleators, Biogeosciences, 20, 2805–2812, https://doi.org/10.5194/bg-20-2805-2023, 2023.
Fan, J., Wang, Y., Rosenfeld, D., and Liu, X.: Review of Aerosol–Cloud Interactions: Mechanisms, Significance, and Challenges, J. Atmos. Sci., 73, 4221–4252, https://doi.org/10.1175/jas-d-16-0037.1, 2016.
Fröhlich-Nowoisky, J., Hill, T. C. J., Pummer, B. G., Yordanova, P., Franc, G. D., and Pöschl, U.: Ice nucleation activity in the widespread soil fungus Mortierella alpina, Biogeosciences, 12, 1057–1071, https://doi.org/10.5194/bg-12-1057-2015, 2015.
Fröhlich-Nowoisky, J., Kampf, C. J., Weber, B., Huffman, J. A., Pöhlker, C., Andreae, M. O., Lang-Yona, N., Burrows, S. M., Gunthe, S. S., Elbert, W., Su, H., Hoor, P., Thines, E., Hoffmann, T., Després, V. R., and Pöschl, U.: Bioaerosols in the Earth system: Climate, health, and ecosystem interactions, Atmos. Res., 182, 346–376, https://doi.org/10.1016/j.atmosres.2016.07.018, 2016.
Ganzert, L., Bajerski, F., and Wagner, D.: Bacterial community composition and diversity of five different permafrost-affected soils of Northeast Greenland, FEMS Microbiol. Ecol., 89, 426–441, https://doi.org/10.1111/1574-6941.12352, 2014.
Gute, E., David, R. O., Kanji, Z. A., and Abbatt, J. P. D.: Ice Nucleation Ability of Tree Pollen Altered by Atmospheric Processing, ACS Earth Space Chem., 4, 2312–2319, https://doi.org/10.1021/acsearthspacechem.0c00218, 2020.
Hartmann, M., Blunier, T., Brügger, S. O., Schmale, J., Schwikowski, M., Vogel, A., Wex, H., and Stratmann, F.: Variation of Ice Nucleating Particles in the European Arctic Over the Last Centuries, Geophys. Res. Lett., 46, 4007–4016, https://doi.org/10.1029/2019gl082311, 2019.
Hartmann, M., Gong, X., Kecorius, S., van Pinxteren, M., Vogl, T., Welti, A., Wex, H., Zeppenfeld, S., Herrmann, H., Wiedensohler, A., and Stratmann, F.: Terrestrial or marine – indications towards the origin of ice-nucleating particles during melt season in the European Arctic up to 83.7° N, Atmos. Chem. Phys., 21, 11613–11636, https://doi.org/10.5194/acp-21-11613-2021, 2021.
Hartmann, M., Adachi, K., Eppers, O., Haas, C., Herber, A., Holzinger, R., Hünerbein, A., Jäkel, E., Jentzsch, C., van Pinxteren, M., Wex, H., Willmes, S., and Stratmann, F.: Wintertime Airborne Measurements of Ice Nucleating Particles in the High Arctic: A Hint to a Marine, Biogenic Source for Ice Nucleating Particles, Geophys. Res. Lett., 47, e2020GL087770, https://doi.org/10.1029/2020gl087770, 2020.
Hasholt, B. and Hagedorn, B.: Hydrology and Geochemistry of River-Borne Material in a High Arctic Drainage System, Zackenberg, Northeast Greenland, Arct. Antarct. Alpine Res., 32, 84–94, https://doi.org/10.2307/1552413, 2000.
Hill, T. C. J., DeMott, P. J., Tobo, Y., Fröhlich-Nowoisky, J., Moffett, B. F., Franc, G. D., and Kreidenweis, S. M.: Sources of organic ice nucleating particles in soils, Atmos. Chem. Phys., 16, 7195–7211, https://doi.org/10.5194/acp-16-7195-2016, 2016.
Holland, M. M. and Bitz, C. M.: Polar amplification of climate change in coupled models, Clim. Dynam., 21, 221–232, https://doi.org/10.1007/s00382-003-0332-6, 2003.
Hollesen, J., Elberling, B., and Jansson, P. E.: Future active layer dynamics and carbon dioxide production from thawing permafrost layers in Northeast Greenland, Global Change Biol., 17, 911–926, https://doi.org/10.1111/j.1365-2486.2010.02256.x, 2011.
Huang, S., Hu, W., Chen, J., Wu, Z., Zhang, D., and Fu, P.: Overview of biological ice nucleating particles in the atmosphere, Environ. Int., 146, 106197, https://doi.org/10.1016/j.envint.2020.106197, 2021.
Huffman, J. A., Prenni, A. J., DeMott, P. J., Pöhlker, C., Mason, R. H., Robinson, N. H., Fröhlich-Nowoisky, J., Tobo, Y., Després, V. R., Garcia, E., Gochis, D. J., Harris, E., Müller-Germann, I., Ruzene, C., Schmer, B., Sinha, B., Day, D. A., Andreae, M. O., Jimenez, J. L., Gallagher, M., Kreidenweis, S. M., Bertram, A. K., and Pöschl, U.: High concentrations of biological aerosol particles and ice nuclei during and after rain, Atmos. Chem. Phys., 13, 6151–6164, https://doi.org/10.5194/acp-13-6151-2013, 2013.
Ickes, L., Porter, G. C. E., Wagner, R., Adams, M. P., Bierbauer, S., Bertram, A. K., Bilde, M., Christiansen, S., Ekman, A. M. L., Gorokhova, E., Höhler, K., Kiselev, A. A., Leck, C., Möhler, O., Murray, B. J., Schiebel, T., Ullrich, R., and Salter, M. E.: The ice-nucleating activity of Arctic sea surface microlayer samples and marine algal cultures, Atmos. Chem. Phys., 20, 11089–11117, https://doi.org/10.5194/acp-20-11089-2020, 2020.
IPCC: The Earth's Energy Budget, Climate Feedbacks and Climate Sensitivity, in: Climate Change 2021 – The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, edited by: Intergovernmental Panel on Climate, C., Cambridge University Press, Cambridge, 923-1054, https://doi.org/10.1017/9781009157896.009, 2023.
Irish, V. E., Elizondo, P., Chen, J., Chou, C., Charette, J., Lizotte, M., Ladino, L. A., Wilson, T. W., Gosselin, M., Murray, B. J., Polishchuk, E., Abbatt, J. P. D., Miller, L. A., and Bertram, A. K.: Ice-nucleating particles in Canadian Arctic sea-surface microlayer and bulk seawater, Atmos. Chem. Phys., 17, 10583–10595, https://doi.org/10.5194/acp-17-10583-2017, 2017.
Irish, V. E., Hanna, S. J., Xi, Y., Boyer, M., Polishchuk, E., Ahmed, M., Chen, J., Abbatt, J. P. D., Gosselin, M., Chang, R., Miller, L. A., and Bertram, A. K.: Revisiting properties and concentrations of ice-nucleating particles in the sea surface microlayer and bulk seawater in the Canadian Arctic during summer, Atmos. Chem. Phys., 19, 7775–7787, https://doi.org/10.5194/acp-19-7775-2019, 2019.
Jayaweera, K. and Flanagan, P.: Investigations on biogenic ice nuclei in the Arctic atmosphere, Geophys. Res. Lett., 9, 94–97, https://doi.org/10.1029/GL009i001p00094, 1981.
Jensen, L. Z., Glasius, M., Gryning, S.-E., Massling, A., Finster, K., and Šantl-Temkiv, T.: Seasonal Variation of the Atmospheric Bacterial Community in the Greenlandic High Arctic Is Influenced by Weather Events and Local and Distant Sources, Front. Microbiol., 13, 909980, https://doi.org/10.3389/fmicb.2022.909980, 2022.
Kanji, Z. A., Ladino, L. A., Wex, H., Boose, Y., Burkert-Kohn, M., Cziczo, D. J., and Krämer, M.: Overview of Ice Nucleating Particles, Meteor. Mon., 58, 1.1–1.33, https://doi.org/10.1175/AMSMONOGRAPHS-D-16-0006.1, 2017.
Keuschnig, C., Vogel, T. M., Barbaro, E., Spolaor, A., Koziol, K., Bjorkman, M. P., Zdanowicz, C., Gallet, J. C., Luks, B., Layton, R., and Larose, C.: Selection processes of Arctic seasonal glacier snowpack bacterial communities, Microbiome, 11, 35, https://doi.org/10.1186/s40168-023-01473-6, 2023.
Kinney, N. L. H., Hepburn, C. A., Gibson, M. I., Ballesteros, D., and Whale, T. F.: High interspecific variability in ice nucleation activity suggests pollen ice nucleators are incidental, Biogeosciences, 21, 3201–3214, https://doi.org/10.5194/bg-21-3201-2024, 2024.
Knackstedt, K. A., Moffett, B. F., Hartmann, S., Wex, H., Hill, T. C. J., Glasgo, E. D., Reitz, L. A., Augustin-Bauditz, S., Beall, B. F. N., Bullerjahn, G. S., Fröhlich-Nowoisky, J., Grawe, S., Lubitz, J., Stratmann, F., and McKay, R. M. L.: Terrestrial Origin for Abundant Riverine Nanoscale Ice-Nucleating Particles, Environ. Sci. Technol., 52, 12358–12367, https://doi.org/10.1021/acs.est.8b03881, 2018.
Koljalg, U., Larsson, K. H., Abarenkov, K., Nilsson, R. H., Alexander, I. J., Eberhardt, U., Erland, S., Hoiland, K., Kjoller, R., Larsson, E., Pennanen, T., Sen, R., Taylor, A. F., Tedersoo, L., Vralstad, T., and Ursing, B. M.: UNITE: a database providing web-based methods for the molecular identification of ectomycorrhizal fungi, New Phytol., 166, 1063–1068, https://doi.org/10.1111/j.1469-8137.2005.01376.x, 2005.
Kunert, A. T., Pöhlker, M. L., Tang, K., Krevert, C. S., Wieder, C., Speth, K. R., Hanson, L. E., Morris, C. E., Schmale III, D. G., Pöschl, U., and Fröhlich-Nowoisky, J.: Macromolecular fungal ice nuclei in Fusarium: effects of physical and chemical processing, Biogeosciences, 16, 4647–4659, https://doi.org/10.5194/bg-16-4647-2019, 2019.
Larsen, J. A., Conen, F., and Alewell, C.: Export of ice nucleating particles from a watershed, R. Soc. Open Sci., 4, 170213, https://doi.org/10.1098/rsos.170213, 2017.
Liu, C., Cui, Y., Li, X., and Yao, M.: microeco: an R package for data mining in microbial community ecology, FEMS Microbiol. Ecol., 97, fiaa255, https://doi.org/10.1093/femsec/fiaa255, 2021.
Lorv, J. S., Rose, D. R., and Glick, B. R.: Bacterial ice crystal controlling proteins, Scientifica (Cairo), 2014, 976895, https://doi.org/10.1155/2014/976895, 2014.
Malard, L. A., Anwar, M. Z., Jacobsen, C. S., and Pearce, D. A.: Biogeographical patterns in soil bacterial communities across the Arctic region, FEMS Microbiol. Ecol., 95, fiz128, https://doi.org/10.1093/femsec/fiz128, 2019.
Malard, L. A., Mod, H. K., Guex, N., Broennimann, O., Yashiro, E., Lara, E., Mitchell, E. A. D., Niculita-Hirzel, H., and Guisan, A.: Comparative analysis of diversity and environmental niches of soil bacterial, archaeal, fungal and protist communities reveal niche divergences along environmental gradients in the Alps, Soil Biol. Biochem., 169, 108674, https://doi.org/10.1016/j.soilbio.2022.108674, 2022.
Mankoff, K. D., Noël, B., Fettweis, X., Ahlstrøm, A. P., Colgan, W., Kondo, K., Langley, K., Sugiyama, S., van As, D., and Fausto, R. S.: Greenland liquid water discharge from 1958 through 2019, Earth Syst. Sci. Data, 12, 2811–2841, https://doi.org/10.5194/essd-12-2811-2020, 2020.
Mannisto, M. K., Ahonen, S. H. K., Ganzert, L., Tiirola, M., Stark, S., and Haggblom, M. M.: Bacterial and fungal communities in sub-Arctic tundra heaths are shaped by contrasting snow accumulation and nutrient availability, FEMS Microbiol. Ecol., 100, fiae036, https://doi.org/10.1093/femsec/fiae036, 2024.
Martin, M.: Cutadapt removes adapter sequences from high-throughput sequencing reads, 17, 3, https://doi.org/10.14806/ej.17.1.200, 2011.
McMurdie, P. J. and Holmes, S.: phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data, PLOS ONE, 8, e61217, https://doi.org/10.1371/journal.pone.0061217, 2013.
Meinander, O., Dagsson-Waldhauserova, P., Amosov, P., Aseyeva, E., Atkins, C., Baklanov, A., Baldo, C., Barr, S. L., Barzycka, B., Benning, L. G., Cvetkovic, B., Enchilik, P., Frolov, D., Gassó, S., Kandler, K., Kasimov, N., Kavan, J., King, J., Koroleva, T., Krupskaya, V., Kulmala, M., Kusiak, M., Lappalainen, H. K., Laska, M., Lasne, J., Lewandowski, M., Luks, B., McQuaid, J. B., Moroni, B., Murray, B., Möhler, O., Nawrot, A., Nickovic, S., O’Neill, N. T., Pejanovic, G., Popovicheva, O., Ranjbar, K., Romanias, M., Samonova, O., Sanchez-Marroquin, A., Schepanski, K., Semenkov, I., Sharapova, A., Shevnina, E., Shi, Z., Sofiev, M., Thevenet, F., Thorsteinsson, T., Timofeev, M., Umo, N. S., Uppstu, A., Urupina, D., Varga, G., Werner, T., Arnalds, O., and Vukovic Vimic, A.: Newly identified climatically and environmentally significant high-latitude dust sources, Atmos. Chem. Phys., 22, 11889–11930, https://doi.org/10.5194/acp-22-11889-2022, 2022.
Moffett, B., Hill, T., and DeMott, P.: Abundance of Biological Ice Nucleating Particles in the Mississippi and Its Major Tributaries, Atmosphere, 9, 307, https://doi.org/10.3390/atmos9080307, 2018.
Moffett, B. F.: Fresh water ice nuclei, Fundament. Appl. Limnol., 188, 19–23, https://doi.org/10.1127/fal/2016/0851, 2016.
Moffett, B. F., Getti, G., Henderson-Begg, S. K., and Hill, T. C. J.: Ubiquity of ice nucleation in lichen – possible atmospheric implications, Lindbergia, 3, 39–43, https://doi.org/10.25227/linbg.01070, 2015.
Möhler, O., DeMott, P. J., Vali, G., and Levin, Z.: Microbiology and atmospheric processes: the role of biological particles in cloud physics, Biogeosciences, 4, 1059–1071, https://doi.org/10.5194/bg-4-1059-2007, 2007.
Morris, C. E., Kinkel, L. L., Xiao, K., Prior, P., and Sands, D. C.: Surprising niche for the plant pathogen Pseudomonas syringae, Infect. Genet. Evol., 7, 84–92, https://doi.org/10.1016/j.meegid.2006.05.002, 2007.
Morris, C. E., Sands, D. C., Glaux, C., Samsatly, J., Asaad, S., Moukahel, A. R., Gonçalves, F. L. T., and Bigg, E. K.: Urediospores of rust fungi are ice nucleation active at >−10 °C and harbor ice nucleation active bacteria, Atmos. Chem. Phys., 13, 4223–4233, https://doi.org/10.5194/acp-13-4223-2013, 2013.
Morrison, H., de Boer, G., Feingold, G., Harrington, J., Shupe, M. D., and Sulia, K.: Resilience of persistent Arctic mixed-phase clouds, Nat. Geosci., 5, 11–17, https://doi.org/10.1038/ngeo1332, 2011.
Murray, B. J., O'Sullivan, D., Atkinson, J. D., and Webb, M. E.: Ice nucleation by particles immersed in supercooled cloud droplets, Chem. Soc. Rev., 41, 6519–6554, https://doi.org/10.1039/c2cs35200a, 2012.
Nguyen, N. H., Song, Z., Bates, S. T., Branco, S., Tedersoo, L., Menke, J., Schilling, J. S., and Kennedy, P. G.: FUNGuild: An open annotation tool for parsing fungal community datasets by ecological guild, Fungal Ecol., 20, 241–248, https://doi.org/10.1016/j.funeco.2015.06.006, 2016.
O'Sullivan, D., Murray, B. J., Ross, J. F., Whale, T. F., Price, H. C., Atkinson, J. D., Umo, N. S., and Webb, M. E.: The relevance of nanoscale biological fragments for ice nucleation in clouds, Sci. Rep., 5, 8082, https://doi.org/10.1038/srep08082, 2015.
O'Sullivan, D., Murray, B. J., Ross, J. F., and Webb, M. E.: The adsorption of fungal ice-nucleating proteins on mineral dusts: a terrestrial reservoir of atmospheric ice-nucleating particles, Atmos. Chem. Phys., 16, 7879–7887, https://doi.org/10.5194/acp-16-7879-2016, 2016.
Ohkuma, M. and Kudo, T.: Phylogenetic analysis of the symbiotic intestinal microflora of the termite Cryptotermes domesticus, FEMS Microbiol. Lett., 164, 389–395, https://doi.org/10.1111/j.1574-6968.1998.tb13114.x, 1998.
Oksman, M., Kvorning, A. B., Larsen, S. H., Kjeldsen, K. K., Mankoff, K. D., Colgan, W., Andersen, T. J., Nørgaard-Pedersen, N., Seidenkrantz, M.-S., Mikkelsen, N., and Ribeiro, S.: Impact of freshwater runoff from the southwest Greenland Ice Sheet on fjord productivity since the late 19th century, The Cryosphere, 16, 2471–2491, https://doi.org/10.5194/tc-16-2471-2022, 2022.
Pastor, A., Wu, N., Skovsholt, L. J., and Riis, T.: Biofilm Growth in Two Streams Draining Mountainous Permafrost Catchments in NE Greenland, J. Geophys. Res.-Biogeosci., 125, e2019JG005557, https://doi.org/10.1029/2019jg005557, 2020.
Pereira Freitas, G., Adachi, K., Conen, F., Heslin-Rees, D., Krejci, R., Tobo, Y., Yttri, K. E., and Zieger, P.: Regionally sourced bioaerosols drive high-temperature ice nucleating particles in the Arctic, Nat. Commun., 14, 5997, https://doi.org/10.1038/s41467-023-41696-7, 2023.
Phelps, P., Giddings, T. H., Prochoda, M., and Fall, R.: Release of cell-free ice nuclei by Erwinia herbicola, J. Bacteriol., 167, 496–502, https://doi.org/10.1128/jb.167.2.496-502.1986, 1986.
Porter, G. C. E., Adams, M. P., Brooks, I. M., Ickes, L., Karlsson, L., Leck, C., Salter, M. E., Schmale, J., Siegel, K., Sikora, S. N. F., Tarn, M. D., Vullers, J., Wernli, H., Zieger, P., Zinke, J., and Murray, B. J.: Highly Active Ice-Nucleating Particles at the Summer North Pole, J. Geophys. Res.-Atmos., 127, e2021JD036059, https://doi.org/10.1029/2021JD036059, 2022.
Pouleur, S., Richard, C., Martin, J. G., and Antoun, H.: Ice Nucleation Activity in Fusarium acuminatum and Fusarium avenaceum, Appl. Environ. Microbiol., 58, 2960–2964, https://doi.org/10.1128/aem.58.9.2960-2964.1992, 1992.
Pummer, B. G., Budke, C., Augustin-Bauditz, S., Niedermeier, D., Felgitsch, L., Kampf, C. J., Huber, R. G., Liedl, K. R., Loerting, T., Moschen, T., Schauperl, M., Tollinger, M., Morris, C. E., Wex, H., Grothe, H., Pöschl, U., Koop, T., and Fröhlich-Nowoisky, J.: Ice nucleation by water-soluble macromolecules, Atmos. Chem. Phys., 15, 4077–4091, https://doi.org/10.5194/acp-15-4077-2015, 2015.
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Yarza, P., Peplies, J., and Glöckner, F. O.: The SILVA ribosomal RNA gene database project: improved data processing and web-based tools, Nucleic Acid. Res., 41, D590–D596, https://doi.org/10.1093/nar/gks1219, 2012.
Rantanen, M., Karpechko, A. Y., Lipponen, A., Nordling, K., Hyvärinen, O., Ruosteenoja, K., Vihma, T., and Laaksonen, A.: The Arctic has warmed nearly four times faster than the globe since 1979, Commun. Earth Environ., 3, 168, https://doi.org/10.1038/s43247-022-00498-3, 2022.
Rasmussen, C.: Particle Sizing in Geosciences: Explanation of various techniques and pre-treatments, AU Library Scholarly Publishing Services, https://doi.org/10.7146/aul.374, 2020.
Raymond, P. A., Zappa, C. J., Butman, D., Bott, T. L., Potter, J., Mulholland, P., Laursen, A. E., McDowell, W. H., and Newbold, D.: Scaling the gas transfer velocity and hydraulic geometry in streams and small rivers, Limnol. Oceanogr. Fluid. Environ., 2, 41–53, https://doi.org/10.1215/21573689-1597669, 2012.
Riis, T., Tank, J. L., Holmboe, C. M. H., Giménez-Grau, P., Mastepanov, M., Catalán, N., Stott, D., Hansen, B., Kristiansen, S. M., and Pastor, A.: Links Between Stream Water Nitrogen and Terrestrial Vegetation in Northeast Greenland, J. Geophys. Res.-Biogeosci., 128, e2023JG007688, https://doi.org/10.1029/2023JG007688, 2023a.
Riis, T., Tank, J., Holmboe, C. M. H., Giménez-Grau, P., Mastepanov, M., Catalan, N., Stott, D., Hansen, B., Kristiansen, S. M., and Pastor, A.: Stream water chemistry and landscape characteristics in Zackenberg Valley, NE Greenland summer 2021, PANGAEA [data set], https://doi.org/10.1594/PANGAEA.963212, 2023b.
Santl-Temkiv, T., Amato, P., Casamayor, E. O., Lee, P. K. H., and Pointing, S. B.: Microbial ecology of the atmosphere, FEMS Microbiol. Rev., 46, fuac009, https://doi.org/10.1093/femsre/fuac009, 2022.
Santl-Temkiv, T., Gosewinkel, U., Starnawski, P., Lever, M., and Finster, K.: Aeolian dispersal of bacteria in southwest Greenland: their sources, abundance, diversity and physiological states, FEMS Microbiol. Ecol., 94, fiy031, https://doi.org/10.1093/femsec/fiy031, 2018.
Santl-Temkiv, T., Lange, R., Beddows, D., Rauter, U., Pilgaard, S., Dall'Osto, M., Gunde-Cimerman, N., Massling, A., and Wex, H.: Biogenic Sources of Ice Nucleating Particles at the High Arctic Site Villum Research Station, Environ. Sci. Technol., 53, 10580–10590, https://doi.org/10.1021/acs.est.9b00991, 2019.
Savić, S. and Tibell, L.: Atla, a new genus in the Verrucariaceae (Verrucariales), The Lichenologist, 40, 269–282, https://doi.org/10.1017/s0024282908007512, 2008.
Schmid, D., Pridmore, D., Capitani, G., Battistutta, R., Neeser, J. R., and Jann, A.: Molecular organisation of the ice nucleation protein InaV from Pseudomonas syringae, FEBS Lett., 414, 590–594, https://doi.org/10.1016/s0014-5793(97)01079-x, 1997.
Schnell, R. C. and Vali, G.: Biogenic Ice Nuclei: Part I. Terrestrial and Marine Sources, J. Atmos. Sci., 33, 1554–1564, https://doi.org/10.1175/1520-0469(1976)033<1554:BINPIT>2.0.CO;2, 1976.
Schwidetzky, R., de Almeida Ribeiro, I., Bothen, N., Backes, A., DeVries, A. L., Bonn, M., Frhlich-Nowoisky, J., Molinero, V., and Meister, K.: E Pluribus Unum: Functional Aggregation of Cell-Free Proteins Enables Fungal 1 Ice Nucleation, https://doi.org/10.26434/chemrxiv-2023-63qfl-v2, 2023a.
Schwidetzky, R., de Almeida Ribeiro, I., Bothen, N., Backes, A. T., DeVries, A. L., Bonn, M., Frohlich-Nowoisky, J., Molinero, V., and Meister, K.: Functional aggregation of cell-free proteins enables fungal ice nucleation, P. Natl. Acad. Sci. USA, 120, e2303243120, https://doi.org/10.1073/pnas.2303243120, 2023b.
Serreze, M. C. and Barry, R. G.: Processes and impacts of Arctic amplification: A research synthesis, Global Planet. Change, 77, 85–96, https://doi.org/10.1016/j.gloplacha.2011.03.004, 2011.
Serreze, M. C. and Francis, J. A.: The Arctic Amplification Debate, Clim. Change, 76, 241–264, https://doi.org/10.1007/s10584-005-9017-y, 2006.
Shi, Y., Xiang, X., Shen, C., Chu, H., Neufeld, J. D., Walker, V. K., and Grogan, P.: Vegetation-associated impacts on arctic tundra bacterial and microeukaryotic communities, Appl. Environ. Microbiol., 81, 492–501, https://doi.org/10.1128/AEM.03229-14, 2015.
Stopelli, E., Conen, F., Guilbaud, C., Zopfi, J., Alewell, C., and Morris, C. E.: Ice nucleators, bacterial cells and Pseudomonas syringae in precipitation at Jungfraujoch, Biogeosciences, 14, 1189–1196, https://doi.org/10.5194/bg-14-1189-2017, 2017.
Stopelli, E., Conen, F., Morris, C. E., Herrmann, E., Bukowiecki, N., and Alewell, C.: Ice nucleation active particles are efficiently removed by precipitating clouds, Sci. Rep., 5, 16433, https://doi.org/10.1038/srep16433, 2015.
Sze, K. C. H., Wex, H., Hartmann, M., Skov, H., Massling, A., Villanueva, D., and Stratmann, F.: Ice-nucleating particles in northern Greenland: annual cycles, biological contribution and parameterizations, Atmos. Chem. Phys., 23, 4741–4761, https://doi.org/10.5194/acp-23-4741-2023, 2023.
Telagathoti, A., Probst, M., and Peintner, U.: Habitat, Snow-Cover and Soil pH, Affect the Distribution and Diversity of Mortierellaceae Species and Their Associations to Bacteria, Front. Microbiol., 12, 669784, https://doi.org/10.3389/fmicb.2021.669784, 2021.
Tesson, S. V. M. and Santl-Temkiv, T.: Ice Nucleation Activity and Aeolian Dispersal Success in Airborne and Aquatic Microalgae, Front. Microbiol., 9, 2681, https://doi.org/10.3389/fmicb.2018.02681, 2018.
Tobo, Y., Adachi, K., DeMott, P. J., Hill, T. C. J., Hamilton, D. S., Mahowald, N. M., Nagatsuka, N., Ohata, S., Uetake, J., Kondo, Y., and Koike, M.: Glacially sourced dust as a potentially significant source of ice nucleating particles, Nat. Geosci., 12, 253–258, https://doi.org/10.1038/s41561-019-0314-x, 2019.
Toju, H., Tanabe, A. S., Yamamoto, S., and Sato, H.: High-coverage ITS primers for the DNA-based identification of ascomycetes and basidiomycetes in environmental samples, PLoS One, 7, e40863, https://doi.org/10.1371/journal.pone.0040863, 2012.
Vali, G.: Quantitative Evaluation of Experimental Results and the Heterogeneous Freezing Nucleation of Supercooled Liquids, J. Atmos. Sci., 28, 402–409, https://doi.org/10.1175/1520-0469(1971)028<0402:Qeoera>2.0.Co;2, 1971.
Vali, G., Christensen, M., Fresh, R. W., Galyan, E. L., Maki, L. R., and Schnell, R. C.: Biogenic Ice Nuclei. Part II: Bacterial Sources, J. Atmos. Sci., 33, 1565–1570, https://doi.org/10.1175/1520-0469(1976)033<1565:Binpib>2.0.Co;2, 1976.
Varsadiya, M., Urich, T., Hugelius, G., and Barta, J.: Fungi in Permafrost-Affected Soils of the Canadian Arctic: Horizon- and Site-Specific Keystone Taxa Revealed by Co-Occurrence Network, Microorganisms, 9, 1943, https://doi.org/10.3390/microorganisms9091943, 2021.
Watanabe, N. M., Southworth, M. W., Warren, G. J., and Wolber, P. K.: Rates of assembly and degradation of bacterial ice nuclei, Molecular Microbiology, 4, 1871–1879, https://doi.org/10.1111/j.1365-2958.1990.tb02036.x, 1990.
Wen, H., Sullivan, P. L., Billings, S. A., Ajami, H., Cueva, A., Flores, A., Hirmas, D. R., Koop, A. N., Murenbeeld, K., Zhang, X., and Li, L.: From Soils to Streams: Connecting Terrestrial Carbon Transformation, Chemical Weathering, and Solute Export Across Hydrological Regimes, Water Resour. Res., 58, e2022WR032314, https://doi.org/10.1029/2022wr032314, 2022.
Wieber, C., Rosenhøj Jeppesen, M., Finster, K., Melvad, C., and Šantl-Temkiv, T.: Micro-PINGUIN: microtiter-plate-based instrument for ice nucleation detection in gallium with an infrared camera, Atmos. Meas. Tech., 17, 2707–2719, https://doi.org/10.5194/amt-17-2707-2024, 2024a.
Wieber, C., Jensen, L. Z., Vergeynst, L., Maire, L., Juul-Pedersen, T., Finster, K., and Šantl-Temkiv, T.: Terrestrial runoff is an important source of biological INPs in Arctic marine systems, EGUsphere [preprint], https://doi.org/10.5194/egusphere-2024-1633, 2024b.
Wilson, T. W., Ladino, L. A., Alpert, P. A., Breckels, M. N., Brooks, I. M., Browse, J., Burrows, S. M., Carslaw, K. S., Huffman, J. A., Judd, C., Kilthau, W. P., Mason, R. H., McFiggans, G., Miller, L. A., Najera, J. J., Polishchuk, E., Rae, S., Schiller, C. L., Si, M., Temprado, J. V., Whale, T. F., Wong, J. P., Wurl, O., Yakobi-Hancock, J. D., Abbatt, J. P., Aller, J. Y., Bertram, A. K., Knopf, D. A., and Murray, B. J.: A marine biogenic source of atmospheric ice-nucleating particles, Nature, 525, 234–238, https://doi.org/10.1038/nature14986, 2015.
Wong, S. K., Cui, Y., Chun, S. J., Kaneko, R., Masumoto, S., Kitagawa, R., Mori, A. S., Lim, A. S., and Uchida, M.: Vegetation as a key driver of the distribution of microbial generalists that in turn shapes the overall microbial community structure in the low Arctic tundra, Environ. Microbiome, 18, 41, https://doi.org/10.1186/s40793-023-00498-6, 2023.